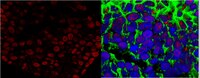
Zebrafish adult-derived hypothalamic neurospheres generate gonadotropin-releasing hormone (GnRH) neurons.
Cortés-Campos, C; Letelier, J; Ceriani, R; Whitlock, KE
Biology open
4
1077-86
2015
Mostrar resumen
Gonadotropin-releasing hormone (GnRH) is a hypothalamic decapeptide essential for fertility in vertebrates. Human male patients lacking GnRH and treated with hormone therapy can remain fertile after cessation of treatment suggesting that new GnRH neurons can be generated during adult life. We used zebrafish to investigate the neurogenic potential of the adult hypothalamus. Previously we have characterized the development of GnRH cells in the zebrafish linking genetic pathways to the differentiation of neuromodulatory and endocrine GnRH cells in specific regions of the brain. Here, we developed a new method to obtain neural progenitors from the adult hypothalamus in vitro. Using this system, we show that neurospheres derived from the adult hypothalamus can be maintained in culture and subsequently differentiate glia and neurons. Importantly, the adult derived progenitors differentiate into neurons containing GnRH and the number of cells is increased through exposure to either testosterone or GnRH, hormones used in therapeutic treatment in humans. Finally, we show in vivo that a neurogenic niche in the hypothalamus contains GnRH positive neurons. Thus, we demonstrated for the first time that neurospheres can be derived from the hypothalamus of the adult zebrafish and that these neural progenitors are capable of producing GnRH containing neurons. | | | 26209533
 |
In vivo reprogrammed pluripotent stem cells from teratomas share analogous properties with their in vitro counterparts.
Choi, HW; Kim, JS; Hong, YJ; Song, H; Seo, HG; Do, JT
Scientific reports
5
13559
2015
Mostrar resumen
Recently, induced pluripotent stem cells (iPSCs) have been generated in vivo from reprogrammable mice. These in vivo iPSCs display features of totipotency, i.e., they differentiate into the trophoblast lineage, as well as all 3 germ layers. Here, we developed a new reprogrammable mouse model carrying an Oct4-GFP reporter gene to facilitate the detection of reprogrammed pluripotent stem cells. Without doxycycline administration, some of the reprogrammable mice developed aggressively growing teratomas that contained Oct4-GFP(+) cells. These teratoma-derived in vivo PSCs were morphologically indistinguishable from ESCs, expressed pluripotency markers, and could differentiate into tissues of all 3 germ layers. However, these in vivo reprogrammed PSCs were more similar to in vitro iPSCs than ESCs and did not contribute to the trophectoderm of the blastocysts after aggregation with 8-cell embryos. Therefore, the ability to differentiate into the trophoblast lineage might not be a unique characteristic of in vivo iPSCs. | | | 26315499
 |
Human Mesenchymal Stem Cells Retain Multilineage Differentiation Capacity Including Neural Marker Expression after Extended In Vitro Expansion.
Okolicsanyi, RK; Camilleri, ET; Oikari, LE; Yu, C; Cool, SM; van Wijnen, AJ; Griffiths, LR; Haupt, LM
PloS one
10
e0137255
2015
Mostrar resumen
The suitability of human mesenchymal stem cells (hMSCs) in regenerative medicine relies on retention of their proliferative expansion potential in conjunction with the ability to differentiate toward multiple lineages. Successful utilisation of these cells in clinical applications linked to tissue regeneration requires consideration of biomarker expression, time in culture and donor age, as well as their ability to differentiate towards mesenchymal (bone, cartilage, fat) or non-mesenchymal (e.g., neural) lineages. To identify potential therapeutic suitability we examined hMSCs after extended expansion including morphological changes, potency (stemness) and multilineage potential. Commercially available hMSC populations were expanded in vitro for greater than 20 passages, equating to greater than 60 days and greater than 50 population doublings. Distinct growth phases (A-C) were observed during serial passaging and cells were characterised for stemness and lineage markers at representative stages (Phase A: P+5, approximately 13 days in culture; Phase B: P+7, approximately 20 days in culture; and Phase C: P+13, approximately 43 days in culture). Cell surface markers, stem cell markers and lineage-specific markers were characterised by FACS, ICC and Q-PCR revealing MSCs maintained their multilineage potential, including neural lineages throughout expansion. Co-expression of multiple lineage markers along with continued CD45 expression in MSCs did not affect completion of osteogenic and adipogenic specification or the formation of neurospheres. Improved standardised isolation and characterisation of MSCs may facilitate the identification of biomarkers to improve therapeutic efficacy to ensure increased reproducibility and routine production of MSCs for therapeutic applications including neural repair. | | | 26356539
 |
Double minute amplification of mutant PDGF receptor α in a mouse glioma model.
Zou, H; Feng, R; Huang, Y; Tripodi, J; Najfeld, V; Tsankova, NM; Jahanshahi, M; Olson, LE; Soriano, P; Friedel, RH
Scientific reports
5
8468
2015
Mostrar resumen
In primary brain tumors, oncogenes are frequently amplified and maintained on extrachromosomal DNA as double minutes (DM), but the underlying mechanisms remain poorly understood. We have generated a mouse model of malignant glioma based on knock-in of a mutant PDGF receptor α (PDGFRα) that is expressed in oligodendrocyte precursor cells (OPCs) after activation by a Cre recombinase. In the tumor suppressor INK4/Arf(-/-) background, mutant animals frequently developed brain tumors resembling anaplastic human gliomas (WHO grade III). Besides brain tumors, most animals also developed aggressive fibrosarcomas, likely triggered by Cre activation of mutant PDGFRα in fibroblastic cell lineages. Importantly, in the brain tumors and cell lines derived from brain tumor tissues, we identified a high prevalence of DM Pdgfra gene amplification, suggesting its occurrence as an early mutational event contributing to the malignant transformation of OPCs. Amplicons extended beyond the Pdgfra locus and included in some cases neighboring genes Kit and Kdr. Our genetically defined mouse brain tumor model therefore supports OPC as a cell of origin for malignant glioma and offers an example of a defined temporal sequence of mutational events, thus providing an entry point for a mechanistic understanding of DM gene amplification and its functionality in gliomagenesis. | | | 25683249
 |
Sox9 is critical for suppression of neurogenesis but not initiation of gliogenesis in the cerebellum.
Vong, KI; Leung, CK; Behringer, RR; Kwan, KM
Molecular brain
8
25
2015
Mostrar resumen
The high mobility group (HMG) family transcription factor Sox9 is critical for induction and maintenance of neural stem cell pool in the central nervous system (CNS). In the spinal cord and retina, Sox9 is also the master regulator that defines glial fate choice by mediating the neurogenic-to-gliogenic fate switch. On the other hand, the genetic repertoire governing the maintenance and fate decision of neural progenitor pool in the cerebellum has remained elusive.By employing the Cre/loxP strategy, we specifically inactivated Sox9 in the mouse cerebellum. Unexpectedly, the self-renewal capacity and multipotency of neural progenitors at the cerebellar ventricular zone (VZ) were not perturbed upon Sox9 ablation. Instead, the mutants exhibited an increased number of VZ-derived neurons including Purkinje cells and GABAergic interneurons. Simultaneously, we observed continuous neurogenesis from Sox9-null VZ at late gestation, when normally neurogenesis ceases to occur and gives way for gliogenesis. Surprisingly, glial cell specification was not affected upon Sox9 ablation.Our findings suggest Sox9 may mediate the neurogenic-to-gliogenic fate switch in mouse cerebellum by modulating the termination of neurogenesis, and therefore indicate a functional discrepancy of Sox9 between the development of cerebellum and other major neural tissues. | | | 25888505
 |
Olig1 function is required for oligodendrocyte differentiation in the mouse brain.
Dai, J; Bercury, KK; Ahrendsen, JT; Macklin, WB
The Journal of neuroscience : the official journal of the Society for Neuroscience
35
4386-402
2015
Mostrar resumen
Oligodendrocyte differentiation and myelination are tightly regulated processes orchestrated by a complex transcriptional network. Two bHLH transcription factors in this network, Olig1 and Olig2, are expressed exclusively by oligodendrocytes after late embryonic development. Although the role of Olig2 in the lineage is well established, the role of Olig1 is still unclear. The current studies analyzed the function of Olig1 in oligodendrocyte differentiation and developmental myelination in brain. Both oligodendrocyte progenitor cell commitment and oligodendrocyte differentiation were impaired in the corpus callosum of Olig1-null mice, resulting in hypomyelination throughout adulthood in the brain. As seen in previous studies with this mouse line, although there was an early myelination deficit in the spinal cord, essentially full recovery with normal spinal cord myelination was seen. Intriguingly, this regional difference may be partially attributed to compensatory upregulation of Olig2 protein expression in the spinal cord after Olig1 deletion, which is not seen in brain. The current study demonstrates a unique role for Olig1 in promoting oligodendrocyte progenitor cell commitment, differentiation, and subsequent myelination primarily in brain, but not spinal cord. | | | 25762682
 |
V-myc immortalizes human neural stem cells in the absence of pluripotency-associated traits.
Pino-Barrio, MJ; García-García, E; Menéndez, P; Martínez-Serrano, A
PloS one
10
e0118499
2015
Mostrar resumen
A better understanding of the molecular mechanisms governing stem cell self-renewal will foster the use of different types of stem cells in disease modeling and cell therapy strategies. Immortalization, understood as the capacity for indefinite expansion, is needed for the generation of any cell line. In the case of v-myc immortalized multipotent human Neural Stem Cells (hNSCs), we hypothesized that v-myc immortalization could induce a more de-differentiated state in v-myc hNSC lines. To test this, we investigated the expression of surface, biochemical and genetic markers of stemness and pluripotency in v-myc immortalized and control hNSCs (primary precursors, that is, neurospheres) and compared these two cell types to human Embryonic Stem Cells (hESCs) and fibroblasts. Using a Hierarchical Clustering method and a Principal Component Analysis (PCA), the v-myc hNSCs associated with their counterparts hNSCs (in the absence of v-myc) and displayed a differential expression pattern when compared to hESCs. Moreover, the expression analysis of pluripotency markers suggested no evidence supporting a reprogramming-like process despite the increment in telomerase expression. In conclusion, v-myc expression in hNSC lines ensures self-renewal through the activation of some genes involved in the maintenance of stem cell properties in multipotent cells but does not alter the expression of key pluripotency-associated genes. | | | 25764185
 |
Non-viral generation of marmoset monkey iPS cells by a six-factor-in-one-vector approach.
Debowski, K; Warthemann, R; Lentes, J; Salinas-Riester, G; Dressel, R; Langenstroth, D; Gromoll, J; Sasaki, E; Behr, R
PloS one
10
e0118424
2015
Mostrar resumen
Groundbreaking studies showed that differentiated somatic cells of mouse and human origin could be reverted to a stable pluripotent state by the ectopic expression of only four proteins. The resulting pluripotent cells, called induced pluripotent stem (iPS) cells, could be an alternative to embryonic stem cells, which are under continuous ethical debate. Hence, iPS cell-derived functional cells such as neurons may become the key for an effective treatment of currently incurable degenerative diseases. However, besides the requirement of efficacy testing of the therapy also its long-term safety needs to be carefully evaluated in settings mirroring the clinical situation in an optimal way. In this context, we chose the long-lived common marmoset monkey (Callithrix jacchus) as a non-human primate species to generate iPS cells. The marmoset monkey is frequently used in biomedical research and is gaining more and more preclinical relevance due to the increasing number of disease models. Here, we describe, to our knowledge, the first-time generation of marmoset monkey iPS cells from postnatal skin fibroblasts by non-viral means. We used the transposon-based, fully reversible piggyback system. We cloned the marmoset monkey reprogramming factors and established robust and reproducible reprogramming protocols with a six-factor-in-one-construct approach. We generated six individual iPS cell lines and characterized them in comparison with marmoset monkey embryonic stem cells. The generated iPS cells are morphologically indistinguishable from marmoset ES cells. The iPS cells are fully reprogrammed as demonstrated by differentiation assays, pluripotency marker expression and transcriptome analysis. They are stable for numerous passages (more than 80) and exhibit euploidy. In summary, we have established efficient non-viral reprogramming protocols for the derivation of stable marmoset monkey iPS cells, which can be used to develop and test cell replacement therapies in preclinical settings. | Immunofluorescence | | 25785453
 |
Repression of Igf1 expression by Ezh2 prevents basal cell differentiation in the developing lung.
Galvis, LA; Holik, AZ; Short, KM; Pasquet, J; Lun, AT; Blewitt, ME; Smyth, IM; Ritchie, ME; Asselin-Labat, ML
Development (Cambridge, England)
142
1458-69
2015
Mostrar resumen
Epigenetic mechanisms involved in the establishment of lung epithelial cell lineage identities during development are largely unknown. Here, we explored the role of the histone methyltransferase Ezh2 during lung lineage determination. Loss of Ezh2 in the lung epithelium leads to defective lung formation and perinatal mortality. We show that Ezh2 is crucial for airway lineage specification and alveolarization. Using optical projection tomography imaging, we found that branching morphogenesis is affected in Ezh2 conditional knockout mice and the remaining bronchioles are abnormal, lacking terminally differentiated secretory club cells. Remarkably, RNA-seq analysis revealed the upregulation of basal genes in Ezh2-deficient epithelium. Three-dimensional imaging for keratin 5 further showed the unexpected presence of a layer of basal cells from the proximal airways to the distal bronchioles in E16.5 embryos. ChIP-seq analysis indicated the presence of Ezh2-mediated repressive marks on the genomic loci of some but not all basal genes, suggesting an indirect mechanism of action of Ezh2. We found that loss of Ezh2 de-represses insulin-like growth factor 1 (Igf1) expression and that modulation of IGF1 signaling ex vivo in wild-type lungs could induce basal cell differentiation. Altogether, our work reveals an unexpected role for Ezh2 in controlling basal cell fate determination in the embryonic lung endoderm, mediated in part by repression of Igf1 expression. | | | 25790853
 |
SOX2 reprograms resident astrocytes into neural progenitors in the adult brain.
Niu, W; Zang, T; Smith, DK; Vue, TY; Zou, Y; Bachoo, R; Johnson, JE; Zhang, CL
Stem cell reports
4
780-94
2015
Mostrar resumen
Glial cells can be in vivo reprogrammed into functional neurons in the adult CNS; however, the process by which this reprogramming occurs is unclear. Here, we show that a distinct cellular sequence is involved in SOX2-driven in situ conversion of adult astrocytes to neurons. This includes ASCL1(+) neural progenitors and DCX(+) adult neuroblasts (iANBs) as intermediates. Importantly, ASCL1 is required, but not sufficient, for the robust generation of iANBs in the adult striatum. These progenitor-derived iANBs predominantly give rise to calretinin(+) interneurons when supplied with neurotrophic factors or the small-molecule valproic acid. Patch-clamp recordings from the induced neurons reveal subtype heterogeneity, though all are functionally mature, fire repetitive action potentials, and receive synaptic inputs. Together, these results show that SOX2-mediated in vivo reprogramming of astrocytes to neurons passes through proliferative intermediate progenitors, which may be exploited for regenerative medicine. | | | 25921813
 |