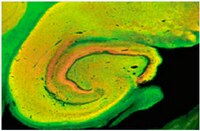
Restraint stress and repeated corticotrophin-releasing factor receptor activation in the amygdala both increase amyloid-β precursor protein and amyloid-β peptide but have divergent effects on brain-derived neurotrophic factor and pre-synaptic proteins in the prefrontal cortex of rats.
Ray, B; Gaskins, DL; Sajdyk, TJ; Spence, JP; Fitz, SD; Shekhar, A; Lahiri, DK
Neuroscience
184
139-50
2010
Mostrar resumen
Both environmental stress and anxiety may represent important risk factors for Alzheimer's disease (AD) pathogenesis. Previous studies demonstrate that restraint stress is associated with increased amyloid beta (Aβ) and decreased brain-derived neurotrophic factor (BDNF) levels in the brain. Aβ deposition, synaptic loss, and neurodegeneration define major hallmarks of AD, and BDNF is responsible for the maintenance of neurons. In contrast to restraint stress, repeated injections of sub-anxiogenic doses of the corticotrophin releasing factor receptor agonist urocortin1 (Ucn1) administered in the basolateral amygdala (BLA) of rats elicits persistent anxiety-like responses. We hypothesized that both restraint stress and Ucn1-induced anxiety would contribute to a neurobiological abnormality that would change the levels of Aβ precursor protein (APP) and Aβ as well as BDNF and pre-synaptic markers. In the first experiment, adult male Wister rats (n=5) were subjected to 3-h restraint, as compared to unstressed controls. In the second experiment, adult male Wistar rats (n=6) were subjected to sub-anxiogenic doses of Ucn1 (6 fmol/100 nl) administered in the BLA for 5 consecutive days, as compared to controls. Following each respective treatment, the social interaction (SI) test was performed to measure anxiety-like behavior. Protein studies were then conducted to quantify levels of APP, Aβ, BDNF and presynaptic proteins in the prefrontal cortex (PFC). In both experiments, we detected differences in either corticosterone levels or the SI test associated with a stress response. Furthermore, our findings indicate that both restraint stress and Ucn1 administration in the BLA lead to increased APP and Aβ deposition. However, restraint-induced stress leads to reductions in the levels of BDNF and presynaptic markers, while Ucn1-induced anxiety is associated with increases in the levels of each respective protein. This demonstrates a convergent role for stress response and Ucn1-induced anxiety in the regulation of APP and Aβ, but opposing roles for each respective treatment in the regulation of BDNF and presynaptic markers. | 21477639
 |
Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model.
Ray, B; Chauhan, NB; Lahiri, DK
Journal of neurochemistry
117
388-402
2010
Mostrar resumen
Alzheimer's disease (AD) is one of the most common forms of dementia in the elderly. In AD patients, β-amyloid peptide (Aβ) plaques and neurofibrillary tangles are common features observed in the CNS. Aβ deposition results in the production of reactive oxygen species (ROS) leading to the hyperphosphorylation of tau that are associated with neuronal damage. Cholinesterase inhibitors and a partial NMDA receptor antagonist (memantine) have been identified as potential treatment options for AD. However, clinical studies have found that these drugs fail to prevent the disease progression. From ancient times, garlic (Allium sativum) has been used to treat several diseases. By 'aging' of garlic, some adverse reactions of garlic can be eliminated. Recent findings suggest that 'aged garlic extract' (AGE) may be a therapeutic agent for AD because of its antioxidant and Aβ lowering properties. To date, the molecular properties of AGE have been sparsely studied in vitro or in vivo. The present study tested specific biochemical and molecular effects of AGE in neuronal and AD rodent models. Furthermore, we identified S-allyl-L-cysteine (SAC) as one of the most active chemicals responsible for the AGE-mediated effect(s). We observed significant neuroprotective and neurorescue properties of AGE and one of its ingredients, SAC, from ROS (H(2)O(2))-mediated insults to neuronal cells. Treatment of AGE and SAC were found to protect neuronal cells when they were independently co-treated with ROS. Furthermore, a novel neuropreservation effect of AGE was detected in that pre-treatment with AGE alone protected ∼ 80% neuronal cells from ROS-mediated damage. AGE was also found to preserve pre-synaptic protein synaptosomal associated protein of 25 kDa (SNAP25) from ROS-mediated insult. For example, treatment with 2% AGE containing diet and SAC (20 mg/kg of diet) independently increased (∼70%) levels of SNAP25 and synaptophysin in Alzheimer's amyloid precursor protein-transgenic mice, of which the latter was significantly decreased in AD. Taken together, the neuroprotective, including preservation of pre-synaptic proteins by AGE and SAC can be utilized in future drug development in AD. | 21166677
 |
The KATP channel activator diazoxide ameliorates amyloid-β and tau pathologies and improves memory in the 3xTgAD mouse model of Alzheimer's disease.
Liu, D; Pitta, M; Lee, JH; Ray, B; Lahiri, DK; Furukawa, K; Mughal, M; Jiang, H; Villarreal, J; Cutler, RG; Greig, NH; Mattson, MP
Journal of Alzheimer's disease : JAD
22
443-57
2009
Mostrar resumen
Compromised cellular energy metabolism, cerebral hypoperfusion, and neuronal calcium dysregulation are involved in the pathological process of Alzheimer's disease (AD). ATP-sensitive potassium (KATP) channels in plasma membrane and inner mitochondrial membrane play important roles in modulating neuronal excitability, cell survival, and cerebral vascular tone. To investigate the therapeutic potential of drugs that activate KATP channels in AD, we first characterized the effects of the KATP channel opener diazoxide on cultured neurons, and then determined its ability to modify the disease process in the 3xTgAD mouse model of AD. Plasma and mitochondrial membrane potentials, cell excitability, intracellular Ca2+ levels and bioenergetics were measured in cultured cerebral cortical neurons exposed to diazoxide. Diazoxide hyperpolarized neurons, reduced the frequency of action potentials, attenuated Ca2+ influx through NMDA receptor channels, and reduced oxidative stress. 3xTgAD mice treated with diazoxide for 8 months exhibited improved performance in a learning and memory test, reduced levels of anxiety, decreased accumulation of Aβ oligomers and hyperphosphorylated tau in the cortex and hippocampus, and increased cerebral blood flow. Our findings show that diazoxide can ameliorate molecular, cytopathological, and behavioral alterations in a mouse model of AD suggesting a therapeutic potential for drugs that activate KATP channels in the treatment of AD. | 20847430
 |
A novel effect of rivastigmine on pre-synaptic proteins and neuronal viability in a neurodegeneration model of fetal rat primary cortical cultures and its implication in Alzheimer's disease.
Bailey, JA; Lahiri, DK
Journal of neurochemistry
112
843-53
2009
Mostrar resumen
Alzheimer's disease (AD) is characterized by deposition of amyloid-beta peptide plaque, disrupted amyloid-beta-precursor protein (APP) metabolism, hyperphosphorylation of Tau leading to neurofibrillary tangles and associated neurotoxicity. Moreover, there is synaptic loss in AD, which occurs early and may precede frank amyloidosis. The central cholinergic system is especially vulnerable to the toxic events associated with AD, and reduced acetylcholine levels in specific brain regions is thought to be central to memory deficits in AD. First-generation cholinesterase inhibitors have provided only symptomatic relief to patients with AD by prolonging the action of remaining acetylcholine with little or no change in the course of the disease. Some second-generation cholinesterase inhibitors are multifunctional drugs that may provide more than purely palliative results. To evaluate the effects of the dual acetylcholinesterase and butyrylcholinesterase inhibitor rivastigmine on key aspects of AD, embryonic day 16 rat primary cortical cultures were treated with rivastigmine under media conditions observed to induce time-dependent neurodegeneration. Samples were subjected to western blotting and immunocytochemistry techniques to determine what influence this drug may have on synaptic proteins and neuronal morphology. There was a strong increase in relative cell viability associated with rivastigmine treatment. Significant dose-dependent increases were observed in the levels of synaptic markers synaptosomal-associated protein of 25 kDa (SNAP-25) and synaptophysin, as well as the neuron-specific form of enolase. Together with an observed enhancement of neuronal morphology, our results suggest a rivastigmine-mediated novel neuroprotective and/or neurorestorative effects involving the synapse. Our observations may explain the potential for rivastigmine to alter the course of AD, and warrant further investigations into using butyrylcholinesterase inhibition as a therapeutic strategy for AD, especially with regard to restoration of synaptic function. | 19912467
 |
Heterogeneous expression of SNAP-25 in rat and human brain.
Garbelli, R; Inverardi, F; Medici, V; Amadeo, A; Verderio, C; Matteoli, M; Frassoni, C
The Journal of comparative neurology
506
373-86
2008
Mostrar resumen
Synaptosomal associated protein of 25 kDa (SNAP-25) is a SNARE component of the exocytotic apparatus involved in the release of neurotransmitter. We used multiple-labeling immunofluorescence, confocal microscopy, and ultrastructural immunocytochemistry to examine the expression of SNAP-25 in excitatory and inhibitory terminals from different rat and human brain areas. Glutamatergic and GABAergic terminals were identified by staining for the vesicular glutamate transporter (vGLUT1), glutamic acid decarboxylase (GAD67), or the vesicular GABA transporter (vGAT). In all examined areas GABAergic terminals did not display detectable levels of SNAP-25, whereas glutamatergic terminals expressed the protein to a variable extent. Codistribution analysis revealed a high colocalization between pixels detecting SNAP-25 labeling and pixels detecting vGLUT1 immunoreactivity. On the contrary, a low degree of pixel colocalization, comparable to that between two unrelated antigens, was detected between SNAP-25 and vGAT, thus suggesting a random overlap of immunofluorescence signals. Our immunofluorescence evidence was supported by ultrastructural data, which clearly confirmed that SNAP-25 was undetectable in GABAergic terminals identified by both their typical morphology and specific staining for GABA. Interestingly, our ultrastructural results confirmed that a subset of glutamatergic synapses do not contain detectable levels of SNAP-25. The present study extends our previous findings obtained in rodent hippocampus and provides evidence that SNAP-25 expression is highly variable between different axon terminals both in rat and human brain. The heterogeneous distribution of SNAP-25 may have important implications not only in relation to the function of the protein as a SNARE but also in the control of network excitability. | 18041776
 |
Elevated synaptophysin I in the prefrontal cortex of human chronic alcoholics.
Henriksson, R; Kuzmin, A; Okvist, A; Harper, C; Sheedy, D; Garrick, T; Yakovleva, T; Bakalkin, G
Synapse (New York, N.Y.)
62
829-33
2008
Mostrar resumen
Convergent lines of evidence suggest potentiation of glutamatergic synapses after chronic ethanol exposure, and indicate that the presynaptic effect hereof is on modulators of synaptic strength rather than on executors of glutamate release. To address this hypothesis in the context of ethanol dependence in humans, we used semiquantitative immunoblotting to compare the immunoreactivities of synaptophysin I, syntaxin 1A, synaptosome-associated protein 25, and vesicle-associated membrane protein in the prefrontal and motor cortices between chronic alcoholics and control subjects. We found a region-specific elevation in synaptophysin I immunoreactivity in the prefrontal cortex of alcoholics, but detected no significant differences between the groups in the immunoreactivities of the other three proteins. Our findings are consistent with an effect of repeated ethanol exposure on modulators of synaptic strength but not on executors of glutamate release, and suggest a role for synaptophysin I in the enduring neuroplasticity in the prefrontal cortical glutamate circuitry that is associated with ethanol dependence. | 18720419
 |
Calpain activity contributes to the control of SNAP-25 levels in neurons.
Grumelli, C; Berghuis, P; Pozzi, D; Caleo, M; Antonucci, F; Bonanno, G; Carmignoto, G; Dobszay, MB; Harkany, T; Matteoli, M; Verderio, C
Molecular and cellular neurosciences
39
314-23
2008
Mostrar resumen
Calpains are a family of calcium-dependent proteases with abundant expression in the CNS, and potent in cleaving some synaptic components. Assessment of calpain activity by its fluorescent substrate, Boc-Leu-Met-CMAC, revealed that cultured neurons display a significant level of constitutive enzyme activity. Notably, calpain activity differs in distinct neuronal populations, with a significantly higher level of activity in GABAergic cells. Using selectively-enriched cultures of fast-spiking GABAergic interneurons, we show that calpain activity partially contributes to the post-translational down regulation of SNAP-25, a calpain substrate, in differentiated GABA cells. In addition, we demonstrate that SNAP-25 is cleaved by calpain in response to acute seizures induced by intraperitoneal kainate injection in vivo. These data indicate that calpains in neurons are active even at physiological calcium concentrations and that different levels of calpain activation in selected neuron subtypes may contribute to the pattern of synaptic protein expression. | 18721885
 |
Autoregulation in PC12 cells via P2Y receptors: Evidence for non-exocytotic nucleotide release from neuroendocrine cells.
Hussl, S; Kubista, H; Boehm, S
Purinergic signalling
3
367-75
2007
Mostrar resumen
Nucleotides are released not only from neurons, but also from various other types of cells including fibroblasts, epithelial, endothelial and glial cells. While ATP release from non-neural cells is frequently Ca(2+) independent and mostly non-vesicular, neuronal ATP release is generally believed to occur via exocytosis. To evaluate whether nucleotide release from neuroendocrine cells might involve a non-vesicular component, the autocrine/paracrine activation of P2Y(12) receptors was used as a biosensor for nucleotide release from PC12 cells. Expression of a plasmid coding for the botulinum toxin C1 light chain led to a decrease in syntaxin 1 detected in immunoblots of PC12 membranes. In parallel, spontaneous as well as depolarization-evoked release of previously incorporated [(3)H]noradrenaline from transfected cells was significantly reduced in comparison with the release from untransfected cells, thus indicating that exocytosis was impaired. In PC12 cells expressing the botulinum toxin C1 light chain, ADP reduced cyclic AMP synthesis to the same extent as in non-transfected cells. Likewise, the enhancement of cyclic AMP synthesis either due to the blockade of P2Y(12) receptors or due to the degradation of extracellular neucleotides by apyrase was not different between non-transfected and botulinum toxin C1 light chain expressing cells. However, the inhibition of cyclic AMP synthesis caused by depolarization-evoked release of endogenous nucleotides was either abolished or greatly reduced in cells expressing the botulinum toxin C1 light chain. Together, these results show that spontaneous nucleotide release from neuroendocrine cells may occur independently of vesicle exocytosis, whereas depolarization-evoked nucleotide release relies predominantly on exocytotic mechanisms. Artículo Texto completo | 18404450
 |
Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder.
Scarr, E; Gray, L; Keriakous, D; Robinson, PJ; Dean, B
Bipolar disorders
8
133-43
2005
Mostrar resumen
In order to identify whether the mechanisms associated with neurotransmitter release are involved in the pathologies of bipolar disorder and schizophrenia, levels of presynaptic [synaptosomal-associated protein-25 (SNAP-25), syntaxin, synaptophysin, vesicle-associated membrane protein, dynamin I] and structural (neuronal cell adhesion molecule and alpha-synuclein) neuronal markers were measured in Brodmann's area 9 obtained postmortem from eight subjects with bipolar I disorder (BPDI), 20 with schizophrenia and 20 controls.Determinations of protein levels were carried out using Western blot techniques with specific antibodies. Levels of mRNA were measured using real-time polymerase chain reaction.In BPDI, levels of SNAP-25 (p < 0.01) and synaptophysin (p < 0.05) increased. There were no changes in schizophrenia or any other changes in BPDI. Levels of mRNA for SNAP-25 were decreased in BPDI (p < 0.05).Changes in SNAP-25 and synaptophysin in BPDI suggest that changes in specific neuronal functions could be linked to the pathology of the disorder. | 16542183
 |
Fatal familial insomnia with an unusual prion protein deposition pattern: an autopsy report with an experimental transmission study.
Sasaki, K; Doh-ura, K; Wakisaka, Y; Tomoda, H; Iwaki, T
Neuropathology and applied neurobiology
31
80-7
2004
Mostrar resumen
We recently performed a post-mortem examination on a Japanese patient who had a prion protein gene mutation responsible for fatal familial insomnia (FFI). The patient initially developed cerebellar ataxia, but finally demonstrated insomnia, hyperkinetic delirium, autonomic signs and myoclonus in the late stage of the illness. Histological examination revealed marked neuronal loss in the thalamus and inferior olivary nucleus; however, prion protein (PrP) deposition was not proved in these lesions by immunohistochemistry. Instead, PrP deposition and spongiform change were both conspicuous within the cerebral cortex, whereas particular PrP deposition was also observed within the cerebellar cortex. The abnormal protease-resistant PrP (PrP(res)) molecules in the cerebral cortex of this case revealed PrP(res) type 2 pattern and were compatible with those of FFI cases, but the transmission study demonstrated that a pathogen in this case was different from that in a case with classical FFI. By inoculation with homogenate made from the cerebral cortex, the disease was transmitted to mice, and neuropathological features that were distinguishable from those previously reported were noted. These findings indicate the possibility that a discrete pathogen was involved in the disease in this case. We suggest that not only the genotype of the PrP gene and some other as yet unknown genetic factors, but also the variation in pathogen strains might be responsible for the varying clinical and pathological features of this disease. | 15634234
 |