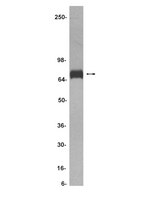
Cripto-1 as a novel therapeutic target for triple negative breast cancer.
Castro, NP; Fedorova-Abrams, ND; Merchant, AS; Rangel, MC; Nagaoka, T; Karasawa, H; Klauzinska, M; Hewitt, SM; Biswas, K; Sharan, SK; Salomon, DS
Oncotarget
6
11910-29
2015
Mostrar resumen
Triple-negative breast cancer (TNBC) presents the poorest prognosis among the breast cancer subtypes and no current standard therapy. Here, we performed an in-depth molecular analysis of a mouse model that establishes spontaneous lung metastasis from JygMC(A) cells. These primary tumors resembled the triple-negative breast cancer (TNBC) both phenotypically and molecularly. Morphologically, primary tumors presented both epithelial and spindle-like cells but displayed only adenocarcinoma-like features in lung parenchyma. The use of laser-capture microdissection combined with Nanostring mRNA and microRNA analysis revealed overexpression of either epithelial and miRNA-200 family or mesenchymal markers in adenocarcinoma and mesenchymal regions, respectively. Cripto-1, an embryonic stem cell marker, was present in spindle-like areas and its promoter showed activity in primary tumors. Cripto-1 knockout by the CRISPR-Cas9 system inhibited tumor growth and pulmonary metastasis. Our findings show characterization of a novel mouse model that mimics the TNBC and reveal Cripto-1 as a TNBC target hence may offer alternative treatment strategies for TNBC. | | 26059540
 |
Tumor-suppressive activity of Lunatic Fringe in prostate through differential modulation of Notch receptor activation.
Zhang, S; Chung, WC; Wu, G; Egan, SE; Xu, K
Neoplasia (New York, N.Y.)
16
158-67
2014
Mostrar resumen
Elevated Notch ligand and receptor expression has been associated with aggressive forms of prostate cancer, suggesting a role for Notch signaling in regulation of prostate tumor initiation and progression. Here, we report a critical role for Lunatic Fringe (Lfng), which encodes an O-fucosylpeptide 3-ß-N-acetylglucosaminyltransferase known to modify epidermal growth factor repeats of Notch receptor proteins, in regulation of prostate epithelial differentiation and proliferation, as well as in prostate tumor suppression. Deletion of Lfng in mice caused altered Notch activation in the prostate, associated with elevated accumulation of Notch1, Notch2, and Notch4 intracellular domains, decreased levels of the putative Notch3 intracellular fragment, as well as increased expression of Hes1, Hes5, and Hey2. Loss of Lfng resulted in expansion of the basal layer, increased proliferation of both luminal and basal cells, and ultimately, prostatic intraepithelial neoplasia. The Lfng-null prostate showed down-regulation of prostatic tumor suppressor gene NKX3.1 and increased androgen receptor expression. Interestingly, expression of LFNG and NKX3.1 were positively correlated in publically available human prostate cancer data sets. Knockdown of LFNG in DU-145 prostate cancer cells led to expansion of CD44(+)CD24(-) and CD49f(+)CD24(-) stem/progenitor-like cell population associated with enhanced prostatosphere-forming capacity. Taken together, these data revealed a tumor-suppressive role for Lfng in the prostate through differential regulation of Notch signaling. | Western Blotting | 24709423
 |
Genome-wide reprogramming of the chromatin landscape underlies endocrine therapy resistance in breast cancer.
Magnani, L; Stoeck, A; Zhang, X; Lánczky, A; Mirabella, AC; Wang, TL; Gyorffy, B; Lupien, M
Proceedings of the National Academy of Sciences of the United States of America
110
E1490-9
2013
Mostrar resumen
The estrogen receptor (ER)α drives growth in two-thirds of all breast cancers. Several targeted therapies, collectively termed endocrine therapy, impinge on estrogen-induced ERα activation to block tumor growth. However, half of ERα-positive breast cancers are tolerant or acquire resistance to endocrine therapy. We demonstrate that genome-wide reprogramming of the chromatin landscape, defined by epigenomic maps for regulatory elements or transcriptional activation and chromatin openness, underlies resistance to endocrine therapy. This annotation reveals endocrine therapy-response specific regulatory networks where NOTCH pathway is overactivated in resistant breast cancer cells, whereas classical ERα signaling is epigenetically disengaged. Blocking NOTCH signaling abrogates growth of resistant breast cancer cells. Its activation state in primary breast tumors is a prognostic factor of resistance in endocrine treated patients. Overall, our work demonstrates that chromatin landscape reprogramming underlies changes in regulatory networks driving endocrine therapy resistance in breast cancer. | | 23576735
 |
Notch4 normalization reduces blood vessel size in arteriovenous malformations.
Murphy, PA; Kim, TN; Lu, G; Bollen, AW; Schaffer, CB; Wang, RA
Science translational medicine
4
117ra8
2011
Mostrar resumen
Abnormally enlarged blood vessels underlie many life-threatening disorders including arteriovenous (AV) malformations (AVMs). The core defect in AVMs is high-flow AV shunts, which connect arteries directly to veins, "stealing" blood from capillaries. Here, we studied mouse brain AV shunts caused by up-regulation of Notch signaling in endothelial cells (ECs) through transgenic expression of constitutively active Notch4 (Notch4*). Using four-dimensional two-photon imaging through a cranial window, we found that normalizing Notch signaling by repressing Notch4* expression converted large-caliber, high-flow AV shunts to capillary-like vessels. The structural regression of the high-flow AV shunts returned blood to capillaries, thus reversing tissue hypoxia. This regression was initiated by vessel narrowing without the loss of ECs and required restoration of EphB4 receptor expression by venous ECs. Normalization of Notch signaling resulting in regression of high-flow AV shunts, and a return to normal blood flow suggests that targeting the Notch pathway may be useful therapeutically for treating diseases such as AVMs. | | 22261032
 |
Elongin C is a mediator of Notch4 activity in human renal tubule cells.
Cummins, TD; Mendenhall, MD; Lowry, MN; Korte, EA; Barati, MT; Khundmiri, SJ; Salyer, SA; Klein, JB; Powell, DW
Biochimica et biophysica acta
1814
1748-57
2010
Mostrar resumen
Notch proteins (Notch 1-4) are a family of trans-membrane cell surface receptors that are converted into transcriptional regulators when activated by interactions with cell surface ligands on adjacent cells. Ligand-binding stimulates proteolytic cleavage of the trans-membrane domain, releasing an active intracellular domain (ICD) that translocates to the nucleus and impacts transcription. In transit, the ICD may interact with regulatory proteins that modulate the expression and transcriptional activity. We have found that Notch4(ICD) expression is enhanced in the tubule cells of fibrotic kidneys from diabetic mice and humans and identified Notch4(ICD) interacting proteins that could be pertinent to normal and pathological functions. Using proteomic techniques, several components of the Elongin C complex were identified as candidate Notch4(ICD) interactors. Elongin C complexes can function as ubiquitin ligases capable of regulating proteasomal degradation of specific protein substrates. Our studies indicate that ectopic Elongin C expression stimulates Notch4(ICD) degradation and inhibits its transcriptional activity in human kidney tubule HK11 cells. Blocking Elongin C mediated degradation by MG132 indicates the potential for ubiquitin-mediated Elongin C regulation of Notch4(ICD). Functional interaction of Notch4(ICD) and Elongin C provides novel insight into regulation of Notch signaling in epithelial cell biology and disease. | Western Blotting | 22001063
 |
Transforming acidic coiled-coil protein-3 (Tacc3) acts as a negative regulator of Notch signaling through binding to CDC10/Ankyrin repeats.
Sharon Bargo,Ahmed Raafat,David McCurdy,Idean Amirjazil,Youmin Shu,June Traicoff,Joshua Plant,Barbara K Vonderhaar,Robert Callahan
Biochemical and biophysical research communications
400
2009
Mostrar resumen
We have identified the transforming acidic coiled-coil protein-3 (Tacc3) as a binding partner for Notch4/Int3 and were able to show that it binds to the intracellular domain (ICD) of all members of the Notch receptor family. Members of the Tacc family reside at the centrosomes and associates with microtubules. Recent studies suggest that Tacc3 also contributes to the regulation of gene transcription. Tacc3 specifically interacts with the Notch4/Int3 CDC10/Ankyrin repeats and to a lesser extent, with residues C-terminal to these repeats in the ICD. Dual label immunofluorescence of mouse mammary tissue shows Tacc3 co-localizes with the Notch3 ICD. Co-immunoprecipitation of endogenous Notch and Tacc3 proteins from NIH3T3 cell extracts, lung and mammary gland confirms that these two proteins interact under physiological conditions. In addition, knock down of Tacc3 in NIH3T3 cells leads to the up-regulation of Hey2, a target gene for Notch signaling. The affinity of Tacc3 binding to Notch4/Int3 ICD is similar to that between Rbpj and Notch4/Int3 ICD. Notch4/Int3 ICD-Tacc3 interaction results in the inhibition of transcription from a Hes1-Luciferase reporter vector in COS-1 cells. The inhibition was reversed in these cells by increasing the levels of Rbpj. Taken together, these results suggest that Tacc3 is a negative regulator of the Notch signaling pathway. Artículo Texto completo | | 20804727
 |
Glucocorticoid and growth factor synergism requirement for Notch4 chromatin domain activation.
Wu, J; Bresnick, EH
Molecular and cellular biology
27
2411-22
2007
Mostrar resumen
The Notch signaling pathway modulates cell fate in diverse contexts, including vascular development. Notch4 is selectively expressed in vascular endothelium and regulates vascular remodeling. The signal-dependent transcription factor activator protein 1 (AP-1) activates Notch4 transcription in endothelial cells, but other factors/signals that regulate Notch4 are largely unknown. We demonstrate that, unlike the established transrepression mechanism in which the glucocorticoid receptor (GR) antagonizes AP-1, AP-1 and GR synergistically activated Notch4 transcription in endothelial cells. Fibroblast growth factor 2 (FGF-2) and cortisol induced AP-1 and GR occupancy, respectively, at a Notch4 promoter composite response element consisting of an imperfect half-glucocorticoid response element and an AP-1 motif, which mediated signal-dependent activation. Analysis of Notch4 promoter complex assembly provided evidence that GR and AP-1 independently occupy the composite response element, but AP-1 stabilizes GR occupancy. In multipotent 10T1/2 cells, FGF-2 and cortisol induced a histone modification pattern at the Notch4 locus mimicking that present in endothelial cells and reprogrammed Notch4 from a repressed to an active state. These results establish the molecular basis for a novel AP-1/GR-Notch4 axis in vascular endothelium. | Western Blotting | 17220278
 |