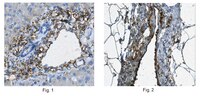
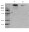
Disulfide-bonded multimers of proteoglycan 4 PRG4 are present in normal synovial fluids.
Schmidt, TA; Plaas, AH; Sandy, JD
Biochimica et biophysica acta
1790
375-84
2009
Mostrar resumen
The proteoglycan 4 (PRG4) gene encodes for a mucin-like O-linked glycosylated protein with several names, including lubricin and superficial zone protein. The objective of this study was to analyze PRG4 in normal bovine calf and steer synovial fluids for evidence of native multimers formed by intermolecular disulfide bonds.A combination of mucin biochemical techniques, with antibodies to both terminal domains and the mucin-like domain of PRG4, were used for analyses.Multimers were present in both calf and steer fluids, and reduction and alkylation converts the multimeric complex (likely dimeric) into monomeric subunits. Tandem mass spectrometry analyses supported the Western blot data and identified PRG4 in the reduced approximately 345 kDa monomeric form. Interestingly, approximately 70 kDa fragments released upon reduction contained peptides from both the N and C terminal regions, which most likely represent fragments of a sparsely glycosylated PRG4 population that are disulfide-linked to extensively glycosylated, intact monomers.The analyses described here have demonstrated the presence of native disulfide-bonded multimers of PRG4 in normal bovine synovial fluids.These structures are similar to those described for multimerization of mucins in general. Such multimerization and proteolytic cleavage of PRG4 may have functional significance in joint health and disease. | 19332105
 |