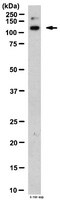
Expression of integrin alpha 10 is transcriptionally activated by pRb in mouse osteoblasts and is downregulated in multiple solid tumors.
Engel, BE; Welsh, E; Emmons, MF; Santiago-Cardona, PG; Cress, WD
Cell death & disease
4
e938
2013
Mostrar resumen
pRb is known as a classic cell cycle regulator whose inactivation is an important initiator of tumorigenesis. However, more recently, it has also been linked to tumor progression. This study defines a role for pRb as a suppressor of the progression to metastasis by upregulating integrin α10. Transcription of this integrin subunit is herein found to be pRb dependent in mouse osteoblasts. Classic pRb partners in cell cycle control, E2F1 and E2F3, do not repress transcription of integrin α10 and phosphorylation of pRb is not necessary for activation of the integrin α10 promoter. Promoter deletion revealed a pRb-responsive region between -108 bp to -55 bp upstream of the start of the site of transcription. pRb activation of transcription also leads to increased levels of integrin α10 protein and a greater concentration of the integrin α10 protein at the cell membrane of mouse osteoblasts. These higher levels of integrin α10 correspond to increased binding to collagen substrate. Consistent with our findings in mouse osteoblasts, we found that integrin α10 is significantly underexpressed in multiple solid tumors that have frequent inactivation of the pRb pathway. Bioinformatically, we identified data consistent with an 'integrin switch' that occurs in multiple solid tumors consisting of underexpression of integrins α7, α8, and α10 with concurrent overexpression of integrin β4. pRb promotes cell adhesion by inducing expression of integrins necessary for cell adhesion to a substrate. We propose that pRb loss in solid tumors exacerbates aggressiveness by debilitating cellular adhesion, which in turn facilitates tumor cell detachment and metastasis. | 24287699
 |