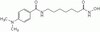
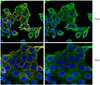
Human umbilical cord mesenchymal stromal cells exhibit immature nucleus pulposus cell phenotype in a laminin-rich pseudo-three-dimensional culture system.
Chon, BH; Lee, EJ; Jing, L; Setton, LA; Chen, J
Stem cell research & therapy
4
120
2013
Mostrar resumen
Cell supplementation to the herniated or degenerated intervertebral disc (IVD) is a potential strategy to promote tissue regeneration and slow disc pathology. Human umbilical cord mesenchymal stromal cells (HUCMSCs) - originating from the Wharton's jelly - remain an attractive candidate for such endeavors with their ability to differentiate into multiple lineages. Previously, mesenchymal stem cells (MSCs) have been studied as a potential source for disc tissue regeneration. However, no studies have demonstrated that MSCs can regenerate matrix with unique characteristics matching that of immature nucleus pulposus (NP) tissues of the IVD. In our prior work, immature NP cells were found to express specific laminin isoforms and laminin-binding receptors that may serve as phenotypic markers for evaluating MSC differentiation to NP-like cells. The goal of this study is to evaluate these markers and matrix synthesis for HUCMSCs cultured in a laminin-rich pseudo-three-dimensional culture system.HUCMSCs were seeded on top of Transwell inserts pre-coated with Matrigel™, which contained mainly laminin-111. Cells were cultured under hypoxia environment with three differentiation conditions: NP differentiation media (containing 2.5% Matrigel™ solution to provide for a pseudo-three-dimensional laminin culture system) with no serum, or the same media supplemented with either insulin-like growth factor-1 (IGF-1) or transforming growth factor-β1 (TGF-β1). Cell clustering behavior, matrix production and the expression of NP-specific laminin and laminin-receptors were evaluated at days 1, 7, 13 and 21 of culture.Data show that a pseudo-three-dimensional culture condition (laminin-1 rich) promoted HUCMSC differentiation under no serum conditions. Starting at day 1, HUCMSCs demonstrated a cell clustering morphology similar to that of immature NP cells in situ and that observed for primary immature NP cells within the similar laminin-rich culture system (prior study). Differentiated HUCMSCs under all conditions were found to contain glycosaminoglycan, expressed extracellular matrix proteins of collagen II and laminin α5, and laminin receptors (integrin α3 and β4 subunits). However, neither growth factor treatment generated distinct differences in NP-like phenotype for HUCMSC as compared with no-serum conditions.HUCMSCs have the potential to differentiate into cells sharing features with immature NP cells in a laminin-rich culture environment and may be useful for IVD cellular therapy. | | 24405888
 |
Palmitoylation by DHHC3 is critical for the function, expression, and stability of integrin α6β4.
Sharma, C; Rabinovitz, I; Hemler, ME
Cellular and molecular life sciences : CMLS
69
2233-44
2011
Mostrar resumen
The laminin-binding integrin α6β4 plays key roles in both normal epithelial and endothelial cells and during tumor cell progression, metastasis, and angiogenesis. Previous cysteine mutagenesis studies have suggested that palmitoylation of α6β4 protein supports a few integrin-dependent functions and molecular associations. Here we took another approach and obtained strikingly different results. We used overexpression and RNAi knockdown in multiple cell types to identify protein acyl transferase DHHC3 as the enzyme responsible for integrin β4 and α6 palmitoylation. Ablation of DHHC3 markedly diminished integrin-dependent cellular cable formation on Matrigel, integrin signaling through Src, and β4 phosphorylation on key diagnostic amino acids (S1356 and 1424). However, unexpectedly, and in sharp contrast to prior α6β4 mutagenesis results, knockdown of DHHC3 accelerated the degradation of α6β4, likely due to an increase in endosomal exposure to cathepsin D. When proteolytic degradation was inhibited (by Pepstatin A), rescued α6β4 accumulated intracellularly, but was unable to reach the cell surface. DHHC3 ablation effects were strongly selective for α6β4. Cell-surface levels of ~10 other proteins (including α3β1) were not diminished, and the appearance of hundreds of other palmitoylated proteins was not altered. Results obtained here demonstrate a new substrate for the DHHC3 enzyme and provide novel opportunities for modulating α6β4 expression, distribution, and function. | | 22314500
 |
Expression of integrin alpha6beta1 enhances tumorigenesis in glioma cells.
Delamarre E, Taboubi S, Mathieu S, Berenguer C, Rigot V, Lissitzky JC, Figarella-Branger D, Ouafik L, Luis J
The American journal of pathology
175
844-855
2009
Mostrar resumen
The integrin alpha6beta1 and its main ligand laminin-111 are overexpressed in glioblastoma, as compared with normal brain tissue, suggesting they-05-be involved in glioblastoma malignancy. To address this question, we stably expressed the alpha6 integrin subunit in the U87 cell line via retroviral-mediated gene transfer. We show that cell surface expression of the alpha6beta1 integrin led to dramatic changes in tumor U87 cell behavior, both in vitro and in vivo. Nude mice receiving either subcutaneous or intracerebral inoculation of alpha6beta1-expressing cells developed substantially more voluminous tumors than mice injected with control cells. The difference in tumor growth was associated with a marked increase in vascularization in response to alpha6beta1 integrin expression and-05-also be related to changes in the balance between cell proliferation and survival. Indeed, expression of alpha6beta1 enhanced proliferation and decreased apoptosis of U87 cells both in the tumor and in vitro. Additionally, we demonstrate that alpha6beta1 is implicated in glioblastoma cell migration and invasion and that laminin-111 might mediate dissemination of alpha6beta1-positive cells in vivo. Our results highlight for the first time the considerable role of the integrin alpha6beta1 in glioma progression., | | 19574430
 |
Expression of laminin isoforms, receptors, and binding proteins unique to nucleus pulposus cells of immature intervertebral disc.
Chen, J; Jing, L; Gilchrist, CL; Richardson, WJ; Fitch, RD; Setton, LA
Connective tissue research
50
294-306
2009
Mostrar resumen
Intervertebral disc (IVD) disorders are believed to be related to aging-related cell loss and phenotypic changes, as well as biochemical and structural changes in the extracellular matrix of the nucleus pulposus (NP) region. Previously, we found that the laminin gamma1 chain was more highly expressed in immature NP porcine tissues, in parallel with the expression pattern for a laminin receptor, integrin alpha6 subunit, as compared to adjacent anulus fibrosus region. This result suggests that cell-matrix interactions may be unique to the immature NP. However, the identity of laminin isoforms specific to immature or mature NP tissues, their associated receptors, and functional significance are still poorly understood. In this study, we evaluated the zonal-specific expression of the laminin chains, receptors (i.e., integrins), and other binding proteins in immature tissue and isolated cells of rat, porcine and human intervertebral disc. Our goal was to reveal features of cellular environment and cell-matrix interactions in the immature NP. Results from both immunohistochemical staining and flow cytometry analysis found that NP cells expressed higher levels of the laminin alpha5 chain, laminin receptors (integrin alpha3, alpha6, beta4 subunit, and CD239), and related binding proteins (CD151), as compared to cells from adjacent anulus fibrosus. These differences suggest that laminin interactions with NP cells are distinct from that of the anulus fibrosus and that laminins may be important contributors to region-specific IVD biology. The revealed laminin isoforms, their receptors, and related binding proteins may be used as distinguishing features of these immature NP cells in the intervertebral disc. | Fluorescence Activated Cell Sorting (FACS), Immunohistochemistry | 19863388
 |
Integrin characterization in pulmonary bronchioles.
Renald A Blundell, David J Harrison
Experimental and molecular pathology
79
74-8
2004
Mostrar resumen
Integrins are a family of cell surface glycoproteins that act as receptors for ECM proteins or for membrane bound counter-receptors on other cells. The integrin receptor family of vertebrates includes at least 16 distinct alpha subunits and at least 8 beta subunits which can associate to form more than 20 distinct integrins. So far, there are no published reports describing integrin characterization in mouse lung tissue and mouse Clara cells. This paper described the characterization of six integrins, mainly alpha(5), alpha(v), alpha(6), beta(1), beta(3), and beta(4), in mouse pulmonary bronchioles and also in Clara cell cultures. alpha(5), alpha(v), alpha(6), beta(1), and beta(4) integrins were present in Clara cells both in tissue sections and cultures. beta(3) integrin was found to be absent in mouse Clara cells. | | 15939420
 |
Upregulation of a functional form of the beta4 integrin subunit in colorectal cancers correlates with c-Myc expression.
Ni, H; Dydensborg, AB; Herring, FE; Basora, N; Gagné, D; Vachon, PH; Beaulieu, JF
Oncogene
24
6820-9
2004
Mostrar resumen
The integrin beta4 subunit has been shown to be involved in various aspects of cancer progression. The aim of the present work was to evaluate the expression of beta4 in primary colon cancers and to investigate the occurrence of a previously identified intestinal nonfunctional variant of beta4 (beta4ctd-) for adhesion to laminin. Immunodetection of beta4 using a panel of antibodies and RT-PCR analyses were performed on series of paired primary colon tumors and corresponding resection margins. The beta4 subunit was found to be significantly overexpressed in cancer specimens at both the protein and transcript levels. Surprisingly, beta4 levels of expression were closely correlated with those of the oncogene c-Myc in individual specimens. In vitro studies of c-Myc overexpression showed an upregulation of beta4 promoter activity. Finally, the beta4ctd- form was identified in the normal proliferative colonic cells but was found to be predominantly absent in colon cancer cells, both in situ and in vitro. We concluded that the beta4ctd- form is lost from colon cancer cells, while the level of the wild-type form of beta4, which is functional for adhesion to laminin, is increased in primary tumors in relation with the expression of c-Myc. | | 16007143
 |
Homeobox D10 induces phenotypic reversion of breast tumor cells in a three-dimensional culture model.
Carrio, M; Arderiu, G; Myers, C; Boudreau, NJ
Cancer research
65
7177-85
2004
Mostrar resumen
Homeobox (Hox) genes are master regulatory genes that direct organogenesis and maintain differentiated tissue function. We previously reported that HoxD10 helps to maintain a quiescent, differentiated phenotype in endothelial cells by suppressing expression of genes involved in remodeling the extracellular matrix and cell migration. Here we investigated whether HoxD10 could also promote or maintain a differentiated phenotype in epithelial cells. We observed that HoxD10 expression is progressively reduced in epithelial cells as malignancy increases in both breast and endometrial tumors. Retroviral gene transfer to restore expression of HoxD10 in the malignant breast tumor cells MDA-MB-231 significantly impaired migration, and when these cells were cultured in a three-dimensional laminin-rich basement membrane (3DlrBM) model, they formed polarized, acinar structures. This phenotypic reversion was accompanied by decreased alpha3 integrin expression and reduced proliferation. Importantly, expression of HoxD10 in the MDA-MB-231 cells inhibited their ability to form tumors in mouse xenografts. Taken together, our results suggest that HoxD10 has tumor-suppressive functions for mammary epithelial cells. | | 16103068
 |
Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream.
Shin-ichi Murase, Alan F Horwitz
The Journal of neuroscience : the official journal of the Society for Neuroscience
22
3568-79
2002
Mostrar resumen
Precursors of the olfactory interneurons migrate from the subventricular zone via the rostral migratory stream (RMS). To investigate the molecular mechanisms by which RMS cells migrate, we used a slice preparation, which allows the migrating cells to be imaged at very high temporal and spatial resolution in the presence of added inhibitors. Using immunohistochemistry, we first determined that the alpha1-, beta8-, and beta1-integrin subunits and the alpha5- and gamma1-laminin subunits are expressed during embryonic day 16 to the early postnatal stage. During early postnatal days, alpha(v)- and beta6-integrins appeared, and their expression persisted throughout adulthood. The migrating cells also expressed the netrin receptors neogenin and Deleted in Colorectal Carcinoma (DCC). Netrin-1 is expressed in olfactory mitral cells. Anti-integrin antibodies inhibited the production of protrusions as well as cellular translocation. In contrast, anti-DCC antibodies primarily altered the direction of the protrusions; consequently, the migration was no longer unidirectional, and the speed was reduced. Thus, the interaction of DCC, possibly through an interaction with netrin-1, contributes to the direction of migration by regulating the formation of directed protrusions. In contrast, the integrins function in production of protrusions and cellular translocation, with different integrins participating at different developmental stages. | | 11978833
 |
A biochemical approach reveals cell-surface molecules utilised by Picornaviridae: Human Parechovirus 1 and Echovirus 1.
K Triantafilou, M Triantafilou
Journal of cellular biochemistry
80
373-81
2001
Mostrar resumen
Although receptor virus interactions of several Picornaviridae have been studied in the past, it is becoming apparent that these interactions might be more complex than previously thought. In this study, we have chosen to identify the cell-surface molecules involved in the infectious cycle of two common human pathogens and members of the Piconaviridae family, Echovirus 1 (Echo1) and Human Parechovirus 1 (HPEV1) also known as Echovirus 22. In order to identify the specific cell-surface protein molecules involved in Echo1 and HPEV1 infectious cycles, we have deviced a method, by which free virions were used as an affinity surface, allowing either Echo1 or HPEV1 to bind to solubilised proteins from cells susceptible to the virus infection. The virus-cell-surface protein complexes were then analysed by SDS-PAGE and two-dimensional electrophoresis. Echo1 was shown to bind to two integrin-like proteins of 150 and 120 kDa. While HPEV1 attached to two integrin-like proteins of 120 and 100 kDa. The identity of these proteins was identified via Western blotting. Thus, overall we can conclusively report that Echo1 utilises integrin alpha2beta1, whereas HPEV1 utilises integrin alphavbeta3 on the cell surface. | | 11135368
 |
Differential expression of integrin mRNAs and proteins during normal rat mammary gland development and in carcinogenesis.
R Y Huang, M M Ip, R Y Huang, M M Ip
Cell and tissue research
303
69-80
2001
Mostrar resumen
A rat model was used to address the roles of integrins in the regulation of normal mammary epithelial cell (MEC) growth and differentiation and in mammary carcinogenesis. The expression of integrins alpha5, alpha6, beta1, and beta4 was examined via Northern and Western blotting analyses in freshly isolated MEC from various postnatal developmental stages. mRNAs for all four integrins were detectable at puberty and were increased during pregnancy. During lactation, the expression of alpha5, alpha6, and beta1 integrin mRNAs reached a peak, whereas that of beta4 integrin decreased dramatically. At day 7 of involution, the levels of all four integrin mRNAs were similar to or slightly higher than that of the pubertal mammary gland. Although alpha5 integrin protein decreased during pregnancy and lactation, beta1 and beta4 integrin proteins had similar profiles as the expression of their respective mRNAs, suggesting that integrin gene expression is regulated at both transcriptional and post-transcriptional levels. All four integrins were heterogeneously expressed in 7,12-dimethylbenz[a]anthracene- and N-nitroso-N-methyl-urea-induced mammary tumors and in RBA and NMU rat mammary tumor cell lines. Adhesion assays showed that isolated MEC interacted with fibronectin to a greater extent than with laminin and collagen I in vitro, and that tumor cells with altered integrin expression exhibited greater adhesive ability to various substrata. Together, our results indicate that alpha5, alpha6, beta1, and beta4 integrins are differentially expressed during normal MEC development and in mammary tumors, supporting the hypothesis that these integrins play important yet complex roles in the mammary gland. | | 11236006
 |