The transcription factors Ik-1 and MZF1 downregulate IGF-IR expression in NPM-ALK⁺ T-cell lymphoma.
Vishwamitra, D; Curry, CV; Alkan, S; Song, YH; Gallick, GE; Kaseb, AO; Shi, P; Amin, HM
Molecular cancer
14
53
2015
Mostrar resumen
The type I insulin-like growth factor receptor (IGF-IR) tyrosine kinase promotes the survival of an aggressive subtype of T-cell lymphoma by interacting with nucleophosmin-anaplastic lymphoma kinase (NPM-ALK) oncogenic protein. NPM-ALK(+) T-cell lymphoma exhibits much higher levels of IGF-IR than normal human T lymphocytes. The mechanisms underlying increased expression of IGF-IR in this lymphoma are not known. We hypothesized that upregulation of IGF-IR could be attributed to previously unrecognized defects that inherently exist in the transcriptional machinery in NPM-ALK(+) T-cell lymphoma.Screening studies showed substantially lower levels of the transcription factors Ikaros isoform 1 (Ik-1) and myeloid zinc finger 1 (MZF1) in NPM-ALK(+) T-cell lymphoma cell lines and primary tumor tissues from patients than in human T lymphocytes. A luciferase assay supported that Ik-1 and MZF1 suppress IGF-IR gene promoter. Furthermore, ChIP assay showed that these transcription factors bind specific sites located within the IGF-IR gene promoter. Forced expression of Ik-1 or MZF1 in the lymphoma cells decreased IGF-IR mRNA and protein. This decrease was associated with downregulation of pIGF-IR, and the phosphorylation of its interacting proteins IRS-1, AKT, and NPM-ALK. In addition, overexpression of Ik-1 and MZF1 decreased the viability, proliferation, migration, and anchorage-independent colony formation of the lymphoma cells.Our results provide novel evidence that the aberrant decreases in Ik-1 and MZF1 contribute significantly to the pathogenesis of NPM-ALK(+) T-cell lymphoma through the upregulation of IGF-IR expression. These findings could be exploited to devise new strategies to eradicate this lymphoma. | | 25884514
 |
Age-related macular degeneration-associated silent polymorphisms in HtrA1 impair its ability to antagonize insulin-like growth factor 1.
Jacobo, SM; Deangelis, MM; Kim, IK; Kazlauskas, A
Molecular and cellular biology
33
1976-90
2013
Mostrar resumen
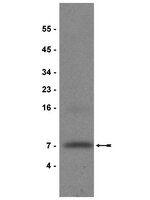
Synonymous single nucleotide polymorphisms (SNPs) within a transcript's coding region produce no change in the amino acid sequence of the protein product and are therefore intuitively assumed to have a neutral effect on protein function. We report that two common variants of high-temperature requirement A1 (HTRA1) that increase the inherited risk of neovascular age-related macular degeneration (NvAMD) harbor synonymous SNPs within exon 1 of HTRA1 that convert common codons for Ala34 and Gly36 to less frequently used codons. The frequent-to-rare codon conversion reduced the mRNA translation rate and appeared to compromise HtrA1's conformation and function. The protein product generated from the SNP-containing cDNA displayed enhanced susceptibility to proteolysis and a reduced affinity for an anti-HtrA1 antibody. The NvAMD-associated synonymous polymorphisms lie within HtrA1's putative insulin-like growth factor 1 (IGF-1) binding domain. They reduced HtrA1's abilities to associate with IGF-1 and to ameliorate IGF-1-stimulated signaling events and cellular responses. These observations highlight the relevance of synonymous codon usage to protein function and implicate homeostatic protein quality control mechanisms that may go awry in NvAMD. | Western Blotting | 23478260
 |
Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury.
Sato, A; Ohtaki, H; Tsumuraya, T; Song, D; Ohara, K; Asano, M; Iwakura, Y; Atsumi, T; Shioda, S
Journal of neuroinflammation
9
65
2011
Mostrar resumen
Microglia and macrophages (MG/MΦ) have a diverse range of functions depending on unique cytokine stimuli, and contribute to neural cell death, repair, and remodeling during central nervous system diseases. While IL-1 has been shown to exacerbate inflammation, it has also been recognized to enhance neuroregeneration. We determined the activating phenotype of MG/MΦ and the impact of IL-1 in an in vivo spinal cord injury (SCI) model of IL-1 knock-out (KO) mice. Moreover, we demonstrated the contribution of IL-1 to both the classical and alternative activation of MG in vitro using an adult MG primary culture.SCI was induced by transection of the spinal cord between the T9 and T10 vertebra in wild-type and IL-1 KO mice. Locomotor activity was monitored and lesion size was determined for 14 days. TNFα and Ym1 levels were monitored to determine the MG/MΦ activating phenotype. Primary cultures of MG were produced from adult mice, and were exposed to IFNγ or IL-4 with and without IL-1β. Moreover, cultures were exposed to IL-4 and/or IL-13 in the presence and absence of IL-1β.The locomotor activity and lesion area of IL-1 KO mice improved significantly after SCI compared with wild-type mice. TNFα production was significantly suppressed in IL-1 KO mice. Also, Ym1, an alternative activating MG/MΦ marker, did not increase in IL-1 KO mice, suggesting that IL-1 contributes to both the classical and alternative activation of MG/MΦ. We treated primary MG cultures with IFNγ or IL-4 in the presence and absence of IL-1β. Increased nitric oxide and TNFα was present in the culture media and increased inducible NO synthase was detected in cell suspensions following co-treatment with IFNγ and IL-1β. Expression of the alternative activation markers Ym1 and arginase-1 was increased after exposure to IL-4 and further increased after co-treatment with IL-4 and IL-1β. The phenotype was not observed after exposure of cells to IL-13.We demonstrate here in in vivo experiments that IL-1 suppressed SCI in a process mediated by the reduction of inflammatory responses. Moreover, we suggest that IL-1 participates in both the classical and alternative activation of MG in in vivo and in vitro systems. | | 22483094
 |
PTH ameliorates acidosis-induced adverse effects in skeletal growth centers: the PTH-IGF-I axis.
Green, J; Goldberg, R; Maor, G
Kidney international
63
487-500
2003
Mostrar resumen
Chronic metabolic acidosis (CMA) exerts profound adverse effects on bone metabolism thereby leading to impaired skeletal linear growth. We have recently shown that CMA in vitro causes distinct morphological changes in skeletal growth centers along with inhibition of endochondral differentiation. In addition, CMA causes an end organ resistance to the anabolic effects of growth hormone (GH) and locally produced insulin-like growth factor-I (IGF-I) in skeletal growth centers. Given the effects of parathyroid hormone (PTH) and PTH related protein (PTHrP) on the development of cartilaginous bone, we sought to determine whether PTH has any effects on the changes induced by CMA in skeletal growth centers. The interaction between PTH and IGF-I in growth centers during neutral or acidic conditions were studied specifically.An in vitro organ culture system using the murine mandibular condyle was employed as a model for endochondral active growth center. Condyles from six-day-old mice were cultured in BGJb medium of either neutral pH (pH approximately 7.4) or acidic pH (pH approximately 7.15) in the presence or absence of 10-10 mol/L [1-34] PTH. After 24, 48, 72 and 96 hours of culture, the condyles were washed, fixed in formaldehyde, and processed for paraffin embedding. Histologic markers of the growth center were assessed. In addition, the protein level and mRNA expression for various markers of cartilage differentiation were evaluated by immunohistochemistry and in situ hybridization, respectively. The abundance and expression levels of IGF-I and IGF-I receptor (IGF-I-R) were assessed also.Following incubation for 72 hours in acidic conditions, there was a marked attenuation of the chondroblastic zone, suggesting a defect in the process of cellular differentiation. Acidosis also down-regulated endochondral differentiation markers (cartilage specific proteoglycans, collagen type II). This was accompanied by a reduction in the expression of IGF-1, IGF-1 receptor and PTH receptors. PTH (10-10 mol/L) added to acidic cultures prevented the adverse effects of CMA on endochondral differentiation and increased the overall condylar growth, when compared to acidic conditions without PTH. PTH also up-regulated its own receptor in control as well as during acidic conditions, and increased the expression levels of IGF-1 and IGF-1 receptor in the acidotic condyle. Acidosis increased the expression of IGF-I binding protein-4 (IGFBP-4, an inhibitor of IGF-I activity), whereas coincubation with PTH during acidic conditions abrogated the up-regulation of IGFBP-4. Addition of a neutralizing antibody to IGF-I-R during PTH treatment under acidic conditions resulted in the abrogation of the ameliorative effect of PTH on endochondral differentiation. The protein kinase C (PKC) signaling pathway was modulated negatively by CMA. However, PTH activated PKC-alpha under both control and acidic conditions. The phorbol ester, PMA (phorbol 12-myristate 13-acetate), a PKC activator, mimicked the effect of PTH on chondrocyte differentiation.Parathyroid hormone at low concentration stimulates the differentiation and proliferation of cartilage cells and prevents the suppressive effect of acidosis on endochondral bone differentiation and on the IGF-I/IGF-I-R system in skeletal growth centers. Increased local production of IGF-I by PTH, which takes place even during acidotic conditions, mediates, at least in part, the ameliorative effect of PTH. Protein kinase C is probably one of the signaling pathways mediating the salutary effects of PTH on chondrocyte differentiation in growth centers. This study lends further credence to the notion that under certain conditions, PTH or PTHrP can exert anabolic effects in the skeleton. These findings may be of clinical-therapeutic significance in children and patients with CMA. | | 12631114
 |
Growth factors and stromal matrix proteins associated with mammographic densities.
Y P Guo, L J Martin, W Hanna, D Banerjee, N Miller, E Fishell, R Khokha, N F Boyd
Cancer epidemiology, biomarkers prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology
10
243-8
2001
Mostrar resumen
Extensive radiologically dense breast tissue is associated with a marked increase in breast cancer risk. To explore the biological basis for this association, we have examined the association of growth factors and stromal matrix proteins in breast tissue with mammographic densities. Ninety-two formalin-fixed paraffin blocks of breast tissues surrounding benign lesions were obtained, half from breasts with little or no density and half from breasts with extensive density, matched for age at biopsy. Sections were stained for cell nuclei, total collagen, the stromal matrix regulatory protein tissue metalloproteinase-3 (TIMP-3), and the growth factors, transforming growth factor-alpha and insulin-like growth factor (IGF-I). The area of immunoreactive staining was measured using quantitative microscopy. Breast tissue from subjects with extensive densities had a greater nuclear area (P = 0.007), as well as larger stained areas of total collagen (P = 0.003), TIMP-3 (P = 0.08), and IGF-I (P = 0.02) when compared with subjects with little breast density. Differences were greater for subjects less than 50 years of age. These data indicate that increased tissue cellularity, greater amounts of collagen, and increased IGF-I and TIMP-3 expression are found in tissue from mammographically dense breasts and suggest mechanisms that may mediate the associated increased risk of breast cancer. | | 11303594
 |
The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells
Manes, S., et al
J Biol Chem, 274:6935-45 (1999)
1998
| Immunoprecipitation | 10066747
 |
Parathyroid hormone-(1-34) enhances aggrecan synthesis via an insulin-like growth factor-I pathway
Harvey, A. K., et al
J Biol Chem, 274:23249-55 (1999)
1998
| Immunoblotting (Western) | 10438499
 |
Sex hormone-induced prostatic carcinogenesis in the noble rat: the role of insulin-like growth factor-I (IGF-I) and vascular endothelial growth factor (VEGF) in the development of prostate cancer.
Y Z Wang, Y C Wong
The Prostate
35
165-77
1998
Mostrar resumen
BACKGROUND: Despite extensive effort, the mechanisms of prostate carcinogenesis are still unknown. We report on a modified method which enabled us to induce a high incidence of prostate carcinogenesis in the Noble rat and examined the role of insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) and their receptors during sex hormone-induced prostate carcinogenesis. METHODS: Noble rats were implanted subcutaneously with a combination of testosterone and estradiol capsules for up to 12 months. Animals were sacrificed starting at 2 months after implantation, and the prostate gland was removed for histopathological and immunohistochemical studies. RESULTS: The results showed that hyperplasia/dysplasia was detected as early as 2 months after treatment, while carcinoma in situ was induced in 4 months and adenocarcinoma in 7 months. Our data suggest that IGF-1, produced by stromal cells in hyperplasia, exerted its effects, through a paracrine mode, on epithelial cells which were IGF-1 receptor (IGF-1R)-positive. The production of IGF-1 appeared to switch to epithelial cells in adenocarcinoma, through which it regulated tumor cell growth via autocrine mode by binding to IGF-1R of carcinoma cells. On the other hand, VEGF was overexpressed in hyperplastic/dysplastic and carcinoma cells, while VEGF-R was detected in endothelial cells. The results suggest that overexpression of VEGF in deranged epithelia and arterial muscle cells may exert its influence on stromal angiogenesis and abnormal growth of prostate gland. CONCLUSIONS: A modified Noble rat model with a high incidence of prostate carcinogenesis has been developed. Using this model, we have further established that IGF-1 and VEGF may be the critical regulators in mediating epithelial-stromal interactions in sex hormone-induced prostate carcinogenesis. | | 9582085
 |
Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells
Ishizuya, T., et al
J Clin Invest, 99:2961-70 (1997)
1997
| Neutralization Assay | 9185520
 |
Cryogenic spinal cord injury induces astrocytic gene expression of insulin-like growth factor I and insulin-like growth factor binding protein 2 during myelin regeneration
Yao, D. L., et al
J Neurosci Res, 40:647-59 (1995)
1994
| Immunohistochemistry (Tissue) | 7541476
 |




















 Antibody[193399-ALL].jpg)