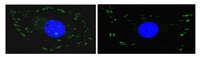
In situ mechanotransduction via vinculin regulates stem cell differentiation.
Holle, AW; Tang, X; Vijayraghavan, D; Vincent, LG; Fuhrmann, A; Choi, YS; del Álamo, JC; Engler, AJ
Stem cells (Dayton, Ohio)
31
2467-77
2013
Mostrar resumen
Human mesenchymal stem cell (hMSC) proliferation, migration, and differentiation have all been linked to extracellular matrix stiffness, yet the signaling pathway(s) that are necessary for mechanotransduction remain unproven. Vinculin has been implicated as a mechanosensor in vitro, but here we demonstrate its ability to also regulate stem cell behavior, including hMSC differentiation. RNA interference-mediated vinculin knockdown significantly decreased stiffness-induced MyoD, a muscle transcription factor, but not Runx2, an osteoblast transcription factor, and impaired stiffness-mediated migration. A kinase binding accessibility screen predicted a cryptic MAPK1 signaling site in vinculin which could regulate these behaviors. Indeed, reintroduction of vinculin domains into knocked down cells indicated that MAPK1 binding site-containing vinculin constructs were necessary for hMSC expression of MyoD. Vinculin knockdown does not appear to interfere with focal adhesion assembly, significantly alter adhesive properties, or diminish cell traction force generation, indicating that its knockdown only adversely affected MAPK1 signaling. These data provide some of the first evidence that a force-sensitive adhesion protein can regulate stem cell fate. | Immunocytochemistry | 23897765
 |
Focal adhesion kinase: in command and control of cell motility.
Mitra, Satyajit K, et al.
Nat. Rev. Mol. Cell Biol., 6: 56-68 (2005)
2004
Mostrar resumen
A central question in cell biology is how membrane-spanning receptors transmit extracellular signals inside cells to modulate cell adhesion and motility. Focal adhesion kinase (FAK) is a crucial signalling component that is activated by numerous stimuli and functions as a biosensor or integrator to control cell motility. Through multifaceted and diverse molecular connections, FAK can influence the cytoskeleton, structures of cell adhesion sites and membrane protrusions to regulate cell movement. | | 15688067
 |
Multiple connections link FAK to cell motility and invasion.
Schlaepfer, David D and Mitra, Satyajit K
Curr. Opin. Genet. Dev., 14: 92-101 (2004)
2004
Mostrar resumen
The ability of intracellular signaling networks to orchestrate a complex biological response such as cell motility requires that individual signaling proteins must act as integrators, responding to multiple extracellular inputs and regulating multiple signaling pathway outputs. In this review, we highlight recent findings that place focal adhesion kinase (FAK) in an important receptor-proximal position in the regulation of growth factor and integrin-stimulated cell motility. Emphasis is placed on the molecular mechanisms of FAK activation, connections of FAK to focal contact formation as well as turnover, and the potential that FAK function in promoting cell invasion may be distinct from its role in cell motility. | | 15108811
 |
Control of motile and invasive cell phenotypes by focal adhesion kinase.
Schlaepfer, David D, et al.
Biochim. Biophys. Acta, 1692: 77-102 (2004)
2004
Mostrar resumen
Cell motility is stimulated by extracellular stimuli and initiated by intracellular signaling proteins that localize to sites of cell contact with the extracellular matrix termed focal contacts. Focal adhesion kinase (FAK) is an intracellular protein-tyrosine kinase (PTK) that acts to regulate the cycle of focal contact formation and disassembly required for efficient cell movement. FAK is activated by a variety of cell surface receptors and transmits signals to a range of targets. Thus, FAK acts as an integrator of cell motility-associated signaling events. We will review the stimulatory and regulatory mechanisms of FAK activation, the different signaling connections of FAK that are mediated by a growing number of FAK-interacting proteins, and the modulation of FAK function by tyrosine and serine phosphorylation. We will also summarize findings with regard to FAK function in vertebrate and invertebrate development as well as recent insights into the mechanistic role(s) of FAK in promoting cell migration. As increased FAK expression and tyrosine phosphorylation have been correlated with the progression to an invasive cell phenotype, there is growing interest in elucidating the important FAK-related signaling connections promoting invasive tumor cell movement. To this end, we will discuss the effects of FAK inhibition via the dominant-negative expression of the FAK C-terminal domain termed FAK-related non-kinase (FRNK) and how these studies have uncovered a distinct role for FAK in promoting cell invasion that may differ from its role in promoting cell motility. | | 15246681
 |
FAK and paxillin: regulators of N-cadherin adhesion and inhibitors of cell migration?
Schaller, Michael D
J. Cell Biol., 166: 157-9 (2004)
2004
Mostrar resumen
FAK and paxillin are important components in integrin-regulated signaling. New evidence suggests that these two proteins function in crosstalk between cell-matrix and cell-cell adhesions. Further, new insight suggests that under some conditions these proteins inhibit cell migration, in contrast to their established roles in several cell systems as positive regulators of cell adhesion and migration. | | 15263014
 |
Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility.
Hanks, Steven K, et al.
Front. Biosci., 8: d982-96 (2003)
2003
Mostrar resumen
Focal adhesion kinase (FAK) was first described in 1992 as a novel nonreceptor protein-tyrosine kinase localized prominently within focal adhesions, suggesting a signaling role in regulating cell behavior resulting from integrin interaction with the extracellular matrix. Subsequent studies over the past decade have established functional roles for FAK as a positive regulator of both cell motility and cell survival, while providing considerable insight into signaling mechanisms involved. FAK signaling results from its ability to become highly phosphorylated in response to integrin-mediated adhesion on Tyr-397, permitting interactions with a number of different signaling effectors containing Src homology 2 (SH2) domains. Src-family kinases recruited to the Tyr-397 site phosphorylate two FAK-interacting proteins, Crk-associated substrate (CAS) and paxillin, which results ultimately in regulation of Rho-family GTPases contributing to cell motility. CAS phosphorylation, as well as phosphatidylinositol 3-kinase (PI3K) activation resulting from its binding to the FAK Tyr-397 site, have been implicated as downstream FAK signaling events that confer a resistance to apoptosis. This article reviews these and other aspects of FAK signaling and function. | | 12700132
 |
Focal adhesion kinase: the first ten years.
Parsons, J Thomas
J. Cell. Sci., 116: 1409-16 (2003)
2003
Mostrar resumen
The protein tyrosine kinase focal adhesion kinase (FAK) plays a prominent role in integrin signaling. FAK activation, demonstrated by an increase in phosphorylation of Tyr397 as well as other sites in the protein, is best understood in the context of the engagement of integrins at the cell surface. Activation of FAK results in recruitment of a number of SH2-domain- and SH3-domain-containing proteins, which mediate signaling to several downstream pathways. FAK-dependent activation of these pathways has been implicated in a diverse array of cellular processes, including cell migration, growth factor signaling, cell cycle progression and cell survival. | | 12640026
 |