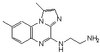
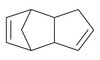
Dvl regulates endo- and exocytotic processes through binding to synaptotagmin.
Kishida, Shosei, et al.
Genes Cells, 12: 49-61 (2007)
2007
Mostrar resumen
Dvl, an important component of the Wnt signalling pathway, is thought to be involved in synaptogenesis. In this study, we investigated whether Dvl regulates neurotransmitter release. Knockdown of Dvl in PC12 cells suppressed K(+)-induced dopamine release, and this phenotype was restored by expression of Dvl-1. We identified synaptotagmin (Syt) I, which is involved in neurotransmitter release, as a Dvl-binding protein. Dvl directly bound to the C2B domain of Syt I. Dvl colocalized with Syt I at the tip of neurites of differentiated PC12 cells and of neurons in the rat dorsal root ganglion. Dvl and Syt I was located in large dense-core vesicles, which contain dopamine. In addition, endocytosis of vesicles containing Syt I was suppressed in Dvl knockdown PC12 cells. Dvl inhibited the binding of Syt I to the complex consisting of syntaxin-1A and SNAP-25. Furthermore, micro2-adaptin of AP-2, which is known to play a role in endocytosis, formed a complex with Dvl and Syt I. Taken together, these results suggest that Dvl is involved in endo- and exocytotic processes through the binding to Syt I. | 17212654
 |
Wnt-3a and Dvl induce neurite retraction by activating Rho-associated kinase.
Kishida, S; Yamamoto, H; Kikuchi, A
Molecular and cellular biology
24
4487-501
2004
Mostrar resumen
Dvl is a key protein that transmits the Wnt signal to the canonical beta-catenin pathway and the noncanonical planar cell polarity (PCP) pathway. We studied the roles of Rho-associated kinase (Rho-kinase), which is activated by Dvl in the PCP pathway of mammalian cells. The expression of Dvl-1, Wnt-1, or Wnt-3a activated Rho-kinase in COS cells, and this activation was inhibited by the Rho-binding domain of Rho-kinase. The expression of Dvl-1 in PC12 cells activated Rho and inhibited nerve growth factor (NGF)-induced neurite outgrowth. This inhibition was reversed by a Rho-kinase inhibitor but not by a c-Jun N-terminal kinase inhibitor. Dvl-1 also inhibited serum starvation-dependent neurite outgrowth of N1E-115 cells, and expression of the Rho-binding domain of Rho-kinase reversed this inhibitory activity of Dvl-1. Dvl-1 mutants that did not activate Rho-kinase did not inhibit the neurite outgrowth of N1E-115 cells. Furthermore, the purified Wnt-3a protein activated Rho-kinase and inhibited the NGF-dependent neurite outgrowth of PC12 cells. Wnt-3a-dependent neurite retraction was also prevented by a Rho-kinase inhibitor and a Dvl-1 mutant that suppresses Wnt-3a-dependent activation of Rho-kinase. These results suggest that Wnt-3a and Dvl regulate neurite formation through Rho-kinase and that PC12 and N1E-115 cells are useful for analyzing the PCP pathway. | 15121866
 |
DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability.
Kishida, S, et al.
Mol. Cell. Biol., 19: 4414-22 (1999)
1998
Mostrar resumen
The N-terminal region of Dvl-1 (a mammalian Dishevelled homolog) shares 37% identity with the C-terminal region of Axin, and this related region is named the DIX domain. The functions of the DIX domains of Dvl-1 and Axin were investigated. By yeast two-hybrid screening, the DIX domain of Dvl-1 was found to interact with Dvl-3, a second mammalian Dishevelled relative. The DIX domains of Dvl-1 and Dvl-3 directly bound one another. Furthermore, Dvl-1 formed a homo-oligomer. Axin also formed a homo-oligomer, and its DIX domain was necessary. The N-terminal region of Dvl-1, including its DIX domain, bound to Axin directly. Dvl-1 inhibited Axin-promoted glycogen synthase kinase 3beta-dependent phosphorylation of beta-catenin, and the DIX domain of Dvl-1 was required for this inhibitory activity. Expression of Dvl-1 in L cells induced the nuclear accumulation of beta-catenin, and deletion of the DIX domain abolished this activity. Although expression of Axin in SW480 cells caused the degradation of beta-catenin and reduced the cell growth rate, expression of an Axin mutant that lacks the DIX domain did not affect the level of beta-catenin or the growth rate. These results indicate that the DIX domains of Dvl-1 and Axin are important for protein-protein interactions and that they are necessary for the ability of Dvl-1 and Axin to regulate the stability of beta-catenin. | 10330181
 |