The early isoform of disabled-1 functions independently of Reelin-mediated tyrosine phosphorylation in chick retina.
Gao, Z; Monckton, EA; Glubrecht, DD; Logan, C; Godbout, R
Molecular and cellular biology
30
4339-53
2009
Mostrar resumen
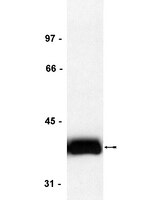
The Reelin-Disabled-1 (Dab1) signaling pathway plays a key role in the positioning of neurons during brain development. Two alternatively spliced Dab1 isoforms have been identified in chick retina and brain: Dab1-E, expressed at early stages of development, and Dab1-L (commonly referred to as Dab1), expressed at later developmental stages. The well-studied Dab1-L serves as an adaptor protein linking Reelin signal to its downstream effectors; however, nothing is known regarding the role of Dab1-E. Here we show that Dab1-E is primarily expressed in proliferating retinal progenitor cells whereas Dab1-L is found exclusively in differentiated neuronal cells. In contrast to Dab1-L, which is tyrosine phosphorylated upon Reelin stimulation, Dab1-E is not tyrosine phosphorylated and may function independently of Reelin. Knockdown of Dab1-E in chick retina results in a significant reduction in the number of proliferating cells and promotes ganglion cell differentiation. Our results demonstrate a role for Dab1-E in the maintenance of the retinal progenitor pool and determination of cell fate. | | 20606009
 |
IL-4 regulates the expression of CD209 and subsequent uptake of Mycobacterium leprae by Schwann cells in human leprosy.
Teles RM, Krutzik SR, Ochoa MT, Oliveira RB, Sarno EN, Modlin RL
Infect Immun
2009
Mostrar resumen
The ability of microbial pathogens to target specific cell types is a key aspect of the pathogenesis of infectious disease. Mycobacterium leprae, by infecting Schwann cells, contributes to nerve injury in patients with leprosy. Here, we investigated mechanisms of host-pathogen interaction in the peripheral nerve lesions of leprosy. We found that the expression of the C-type lectin, CD209, known to be expressed on tissue macrophages and mediate uptake of M. leprae, was present on Schwann cells, colocalizing with the Schwann cell marker, CNPase (2', 3'-cyclic nucleotide 3'-phosphodiesterase), along with the M. leprae antigen PGL-1 in the peripheral nerve biopsy specimens. In vitro, human CD209 positive Schwann cells, both from primary cultures and a long term line, have a higher binding of M. leprae in comparison with CD209 negative Schwann cells. IL-4, known to be expressed in skin lesions from multibacillary patients, increased CD209 expression on human Schwann cells and subsequent Schwann cell binding to M. leprae, while Th1 cytokines did not induce CD209 expression on these cells. Therefore, the regulated expression of CD209 represents a common mechanism by which Schwann cells and macrophages bind and take up M. leprae, contributing to the pathogenesis of leprosy. | | 20713631
 |
SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis.
Stein S, Lohmann C, Schäfer N, Hofmann J, Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Borén J, McBurney MW, Landmesser U, Lüscher TF, Matter CM
Eur Heart J
2009
Mostrar resumen
Aims Endothelial activation, macrophage infiltration, and foam cell formation are pivotal steps in atherogenesis. Our aim in this study was to analyse the role of SIRT1, a class III deacetylase with important metabolic functions, in plaque macrophages and atherogenesis. Methods and results Using partial SIRT1 deletion in atherosclerotic mice, we demonstrate that SIRT1 protects against atherosclerosis by reducing macrophage foam cell formation. Peritoneal macrophages from heterozygous SIRT1 mice accumulate more oxidized low-density lipoprotein (oxLDL), thereby promoting foam cell formation. Bone marrow-restricted SIRT1 deletion confirmed that SIRT1 function in macrophages is sufficient to decrease atherogenesis. Moreover, we show that SIRT1 reduces the uptake of oxLDL by diminishing the expression of lectin-like oxLDL receptor-1 (Lox-1) via suppression of the NF-kappaB signalling pathway. Conclusion Our findings demonstrate protective effects of SIRT1 in atherogenesis and suggest pharmacological SIRT1 activation as a novel anti-atherosclerotic strategy by reducing macrophage foam cell formation. | | 20418343
 |