Use of benchtop exactive high resolution and high mass accuracy orbitrap mass spectrometer for screening in horse doping control.
Yves Moulard,Ludovic Bailly-Chouriberry,Sophie Boyer,Patrice Garcia,Marie-Agnès Popot,Yves Bonnaire
Analytica chimica acta
700
2011
Show Abstract
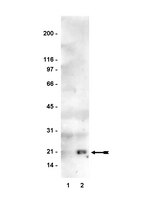
Liquid chromatography-mass spectrometry (LC-MS) has been widely used in doping control laboratories over the last two decades. Currently, simple quadrupole, triple quadrupole and ion trap are the most commonly employed analyzers in toxicological analysis. Nevertheless, the main lack of these technologies is the restricted number of target compounds simultaneously screened without loss of sensitivity. In this article we present an innovative screening approach routinely applied in the French horse doping control laboratory based on high resolution (50000) and high mass accuracy (<5 ppm) in full scan MS mode for more than 235 target analytes screened from an initial volume of 5 mL of urine. The sample preparation was classically founded on solid phase extraction by means of reverse phase C18 cartridges. LC-MS analyses were carried out on a Shimadzu binary HPLC pumps linked to a C18 Sunfire column associated with the high resolution exactive benchtop orbitrap mass spectrometer. This screening was performed alternatively in positive-negative ionization mode during the same run. Thus, the identification of compounds of interest was made using their exact mass in positive-negative ionization mode at their expected retention time. All data obtained were processed by ToxID software (ThermoFisherScientific) which is able to identify a molecule by theoretical mass and retention time. In order to illustrate this innovative technology applied in our laboratory, sample preparation, validation data performed on 20 target compounds from 16 different horse urine samples, chromatograms and spectra will be discussed in this paper. | | 21742125
 |
Salirasib inhibits the growth of hepatocarcinoma cell lines in vitro and tumor growth in vivo through ras and mTOR inhibition.
Charette, N; De Saeger, C; Lannoy, V; Horsmans, Y; Leclercq, I; Stärkel, P
Molecular cancer
9
256
2010
Show Abstract
Dysregulation of epidermal growth factor and insulin-like growth factor signaling play important roles in human hepatocellular carcinoma (HCC), leading to frequent activation of their downstream targets, the ras/raf/extracellular signal-regulated kinase (ERK) and the phosphoinositide 3-kinase (PI3K)/Akt/mammalian Target of Rapamycin (mTOR) pathways. Salirasib is an S-prenyl-cysteine analog that has been shown to block ras and/or mTOR activation in several non hepatic tumor cell lines. We investigated in vitro the effect of salirasib on cell growth as well as its mechanism of action in human hepatoma cell lines (HepG2, Huh7, and Hep3B) and its in vivo effect in a subcutaneous xenograft model with HepG2 cells.Salirasib induced a time and dose dependent growth inhibition in hepatocarcinoma cells through inhibition of proliferation and partially through induction of apoptosis. A 50 percent reduction in cell growth was obtained in all three cell lines at a dose of 150 μM when they were cultured with serum. By contrast, salirasib was more potent at reducing cell growth after stimulation with EGF or IGF2 under serum-free conditions, with an IC50 ranging from 60 μM to 85 μM. The drug-induced anti-proliferative effect was associated with downregulation of cyclin A and to a lesser extent of cyclin D1, and upregulation of p21 and p27. Apoptosis induction was related to a global pro-apoptotic balance with caspase 3 activation, cytochrome c release, death receptor upregulation, and a reduced mRNA expression of the apoptosis inhibitors cFLIP and survivin. These effects were associated with ras downregulation and mTOR inhibition, without reduction of ERK and Akt activation. In vivo, salirasib reduced tumour growth from day 5 onwards. After 12 days of treatment, mean tumor weight was diminished by 56 percent in the treated animals.Our results show for the first time that salirasib inhibits the growth of human hepatoma cell lines through inhibition of proliferation and induction of apoptosis, which is associated with ras and mTOR inhibition. The therapeutic potential of salirasib in human HCC was further confirmed in a subcutaneous xenograft model. Full Text Article | | 20860815
 |
Dependence on PI3K/Akt signaling for malignant rhabdoid tumor cell survival.
Kristen Foster,Yong Wang,Daohong Zhou,Cynthia Wright
Cancer chemotherapy and pharmacology
63
2009
Show Abstract
Malignant rhabdoid tumors (MRT), although rare, are one of the most aggressive pediatric malignancies. Loss of INI1, a tumor suppressor gene and member of the SWI/SNF chromatin remodeling complex, is a recurrent genetic characteristic of these tumors and an important diagnostic marker. We have previously demonstrated a novel interaction between the serine/threonine kinase Akt and INI1, as well as other SWI/SNF subunits. This, coupled with experiments in the literature suggesting that the PI3K/Akt pathway is dysregulated in MRT cells, caused us to investigate the activation and importance of this pathway in this tumor type. Full Text Article | | 18641990
 |
The lutropin/choriogonadotropin receptor-induced phosphorylation of the extracellular signal-regulated kinases in leydig cells is mediated by a protein kinase a-dependent activation of ras
Hirakawa, T. and Ascoli, M.
Mol Endocrinol, 17:2189-200 (2003)
2003
| Activation Assay | 12920236
 |
T cell receptor-mediated signal transduction controlled by the beta chain transmembrane domain: apoptosis-deficient cells display unbalanced mitogen-activated protein kinases activities upon T cell receptor engagement.
Teixeiro, Emma, et al.
J. Biol. Chem., 277: 3993-4002 (2002)
2002
Show Abstract
The bases that support the versatility of the T cell receptor (TCR) to generate distinct T cell responses remain unclear. We have previously shown that mutant cells in the transmembrane domain of TCRbeta chain are impaired in TCR-induced apoptosis but are not affected in other functions. Here we describe the biochemical mechanisms by which this mutant receptor supports some T cell responses but fails to induce apoptosis. Extracellular signal-regulated protein kinase (ERK) is activated at higher and more sustained levels in TCRbeta-mutated than in wild type cells. Conversely, activation of both c-Jun N-terminal kinase and p38 mitogen-activated protein kinase is severely reduced in mutant cells. By attempting to link this unbalanced induction to altered upstream events, we found that ZAP-70 is normally activated. However, although SLP-76 phosphorylation is normally induced, TCR engagement of mutant cells results in lower tyrosine phosphorylation of LAT but in higher tyrosine phosphorylation of Vav than in wild type cells. The results suggest that an altered signaling cascade leading to an imbalance in mitogen-activated protein kinase activities is involved in the selective impairment of apoptosis in these mutant cells. Furthermore, they also provide new insights in the contribution of TCR to decipher the signals that mediate apoptosis distinctly from proliferation. | Protein Binding | 11724779
 |
Eicosanoid activation of extracellular signal-regulated kinase1/2 in human epidermoid carcinoma cells
Szekeres, C. K., et al
J Biol Chem, 275:38831-41 (2000)
2000
| Immunoblotting (Western) | 10952974
 |
The hepatitis B virus HBx protein inhibits caspase 3 activity
Gottlob, K., et al
J Biol Chem, 273:33347-53 (1998)
1998
| Protein Binding | 9837909
 |
Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras.
de Rooij, J and Bos, J L
Oncogene, 14: 623-5 (1997)
1997
Show Abstract
Ras is a small GTPase that cycles between an inactive GDP-bound and an active GTP-bound form. A large variety of ligands that stimulate cell surface receptors induce the activation of Ras. Thus far, this activation could only be measured by the increase of GTP bound to Ras, which was precipitated from radio-labelled cell extract. We have used the minimal Ras-binding domain (RBD) of Raf1 (aa 51-131) to identify in vivo activated Ras. This novel method is based on the observation that RBD binds RasGTP in vitro with a Kd of 20 nM whereas the affinity between RBD and RasGDP is three orders of magnitude lower. Here we show that the Gst-RBD fusion protein precipitates transfected RasL61 (RasGTP) but not RasN17 (RasGDP) from cell lysates. In addition, we demonstrate for two different cell lines that the increase in RasGTP is reflected by an increase in Ras bound to Gst-RBD. From these results we conclude that the minimal Ras-binding domain of Raf1 is an excellent activation specific-probe for Ras. | | 9053862
 |
Biphasic activation of p21ras by endothelin-1 sequentially activates the ERK cascade and phosphatidylinositol 3-kinase.
Foschi, M, et al.
EMBO J., 16: 6439-51 (1997)
1997
Show Abstract
Endothelin-1 (ET-1) induces cell proliferation and differentiation through multiple G-protein-linked signaling systems, including p21ras activation. Whereas p21ras activation and desensitization by receptor tyrosine kinases have been extensively investigated, the kinetics of p21ras activation induced by engagement of G-protein-coupled receptors remains to be fully elucidated. In the present study we show that ET-1 induces a biphasic activation of p21ras in rat glomerular mesangial cells. The first peak of activation of p21ras, at 2-5 min, is mediated by immediate association of phosphorylated Shc with the guanosine exchange factor Sos1 via the adaptor protein Grb2. This initial activation of p21ras results in activation of the extracellular signal-regulated kinase (ERK) cascade. We demonstrate that ET-1 signaling elicits a negative feedback mechanism, modulating p21ras activity through ERK-dependent Sos1 phosphorylation, findings which were confirmed using an adenovirus MEK construct. Subsequent to p21ras and ERK deactivation, Sos1 reverts to the non-phosphorylated condition, enabling it to bind again to the Grb2/Shc complex, which is stabilized by persistent Shc phosphorylation. However, the resulting secondary activation of p21ras at 30 min does not lead to ERK activation, correlating with intensive, ET-1-induced expression of MAP kinase phosphatase-1, but does result in increased p21ras-associated phosphatidylinositol 3-kinase activity. Our data provide evidence that ET-1-induced biphasic p21ras activation causes sequential stimulation of divergent downstream signaling pathways. | | 9351826
 |
Cell cycle-dependent activation of Ras.
Taylor, S J and Shalloway, D
Curr. Biol., 6: 1621-7 (1996)
1996
Show Abstract
BACKGROUND: Ras proteins play an essential role in the transduction of signals from a wide range of cell-surface receptors to the nucleus. These signals may promote cellular proliferation or differentiation, depending on the cell background. It is well established that Ras plays an important role in the transduction of mitogenic signals from activated growth-factor receptors, leading to cell-cycle entry. However, important questions remain as to whether Ras controls signalling events during cell-cycle progression and, if so, at which point in the cell-cycle it is activated. RESULTS: To address these questions we have developed a novel, functional assay for the detection of cellular activated Ras. Using this assay, we found that Ras was activated in HeLa cells, following release from mitosis, and in NIH 3T3 fibroblasts, following serum-stimulated cell-cycle entry. In each case, peak Ras activation occurred in mid-G1 phase. Ras activation in HeLa cells at mid-G1 phase was dependent on RNA and protein synthesis and was not associated with tyrosine phosphorylation of Shc proteins and their binding to Grb2. Significantly, activation of Ras and the extracellular-signal regulated (ERK) sub-group of mitogen-activated protein kinases were not temporally correlated during G1-phase progression. CONCLUSIONS: Activation of Ras during mid-G1 phase appears to differ in many respects from its rapid activation by growth factors, suggesting a novel mechanism of regulation that may be intrinsic to cell-cycle progression. Furthermore, the temporal dissociation between Ras and ERK activation suggests that Ras targets alternate effector pathways during G1-phase progression. | | 8994826
 |