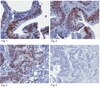
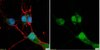
Membrane Type 1 Matrix Metalloproteinase Regulates Monocyte Migration and Collagen Destruction in Tuberculosis.
Sathyamoorthy, T; Tezera, LB; Walker, NF; Brilha, S; Saraiva, L; Mauri, FA; Wilkinson, RJ; Friedland, JS; Elkington, PT
Journal of immunology (Baltimore, Md. : 1950)
195
882-91
2015
Show Abstract
Tuberculosis (TB) remains a global pandemic and drug resistance is rising. Multicellular granuloma formation is the pathological hallmark of Mycobacterium tuberculosis infection. The membrane type 1 matrix metalloproteinase (MT1-MMP or MMP-14) is a collagenase that is key in leukocyte migration and collagen destruction. In patients with TB, induced sputum MT1-MMP mRNA levels were increased 5.1-fold compared with matched controls and correlated positively with extent of lung infiltration on chest radiographs (r = 0.483; p less than 0.05). M. tuberculosis infection of primary human monocytes increased MT1-MMP surface expression 31.7-fold and gene expression 24.5-fold. M. tuberculosis-infected monocytes degraded collagen matrix in an MT1-MMP-dependent manner, and MT1-MMP neutralization decreased collagen degradation by 73%. In human TB granulomas, MT1-MMP immunoreactivity was observed in macrophages throughout the granuloma. Monocyte-monocyte networks caused a 17.5-fold increase in MT1-MMP surface expression dependent on p38 MAPK and G protein-coupled receptor-dependent signaling. Monocytes migrating toward agarose beads impregnated with conditioned media from M. tuberculosis-infected monocytes expressed MT1-MMP. Neutralization of MT1-MMP activity decreased this M. tuberculosis network-dependent monocyte migration by 44%. Taken together, we demonstrate that MT1-MMP is central to two key elements of TB pathogenesis, causing collagen degradation and regulating monocyte migration. | | 26091717
 |
Functional relationship between matrix metalloproteinase-11 and matrix metalloproteinase-14.
Buache, E; Thai, R; Wendling, C; Alpy, F; Page, A; Chenard, MP; Dive, V; Ruff, M; Dejaegere, A; Tomasetto, C; Rio, MC
Cancer medicine
3
1197-210
2014
Show Abstract
MMP-11 is a key factor in physiopathological tissue remodeling. As an active form is secreted, its activity must be tightly regulated to avoid detrimental effects. Although TIMP-1 and TIMP-2 reversibly inhibit MMP-11, another more drastic scenario, presumably via hydrolysis, could be hypothesized. In this context, we have investigated the possible implication of MMP-14, since it exhibits a spatiotemporal localization similar to MMP-11. Using native HFL1-produced MMP-11 and HT-1080-produced MMP-14 as well as recombinant proteins, we show that MMP-11 is a MMP-14 substrate. MMP-14 cleaves MMP-11 catalytic domain at the PGG(P1)-I(P1')LA and V/IQH(P1)-L(P1')YG scissile bonds, two new cleavage sites. Interestingly, a functional test showed a dramatical reduction in MMP-11 enzymatic activity when incubated with active MMP-14, whereas inactive point-mutated MMP-14 had no effect. This function is conserved between human and mouse. Thus, in addition to the canonical reversible TIMP-dependent inhibitory system, irreversible MMP proteolytic inactivation might occur by cleavage of the catalytic domain in a MMP-dependent manner. Since MMP-14 is produced by HT-1080 cancer cells, whereas MMP-11 is secreted by HFL1 stromal cells, our findings support the emerging importance of tumor-stroma interaction/cross-talk. Moreover, they highlight a Janus-faced MMP-14 function in the MMP cascade, favoring activation of several pro-MMPs, but limiting MMP-11 activity. Finally, both MMPs are active at the cell periphery. Since MMP-14 is present at the cell membrane, whereas MMP-11 is soluble into the cellular microenvironment, this MMP-14 function might represent one critical regulatory mechanism to control the extent of pericellular MMP-11 bioavailability and protect cells from excessive/inappropriate MMP-11 function. | | 25081520
 |
Podosomes of dendritic cells facilitate antigen sampling.
Baranov, MV; Ter Beest, M; Reinieren-Beeren, I; Cambi, A; Figdor, CG; van den Bogaart, G
Journal of cell science
127
1052-64
2014
Show Abstract
Dendritic cells sample the environment for antigens and play an important role in establishing the link between innate and acquired immunity. Dendritic cells contain mechanosensitive adhesive structures called podosomes that consist of an actin-rich core surrounded by integrins, adaptor proteins and actin network filaments. They facilitate cell migration via localized degradation of extracellular matrix. Here, we show that podosomes of human dendritic cells locate to spots of low physical resistance in the substrate (soft spots) where they can evolve into protrusive structures. Pathogen recognition receptors locate to these protrusive structures where they can trigger localized antigen uptake, processing and presentation to activate T-cells. Our data demonstrate a novel role in antigen sampling for the podosomes of dendritic cells. | Mouse | 24424029
 |
N-WASP coordinates the delivery and F-actin-mediated capture of MT1-MMP at invasive pseudopods.
Yu, X; Zech, T; McDonald, L; Gonzalez, EG; Li, A; Macpherson, I; Schwarz, JP; Spence, H; Futó, K; Timpson, P; Nixon, C; Ma, Y; Anton, IM; Visegrády, B; Insall, RH; Oien, K; Blyth, K; Norman, JC; Machesky, LM
The Journal of cell biology
199
527-44
2012
Show Abstract
Metastasizing tumor cells use matrix metalloproteases, such as the transmembrane collagenase MT1-MMP, together with actin-based protrusions, to break through extracellular matrix barriers and migrate in dense matrix. Here we show that the actin nucleation-promoting protein N-WASP (Neural Wiskott-Aldrich syndrome protein) is up-regulated in breast cancer, and has a pivotal role in mediating the assembly of elongated pseudopodia that are instrumental in matrix degradation. Although a role for N-WASP in invadopodia was known, we now show how N-WASP regulates invasive protrusion in 3D matrices. In actively invading cells, N-WASP promoted trafficking of MT1-MMP into invasive pseudopodia, primarily from late endosomes, from which it was delivered to the plasma membrane. Upon MT1-MMP's arrival at the plasma membrane in pseudopodia, N-WASP stabilized MT1-MMP via direct tethering of its cytoplasmic tail to F-actin. Thus, N-WASP is crucial for extension of invasive pseudopods into which MT1-MMP traffics and for providing the correct cytoskeletal framework to couple matrix remodeling with protrusive invasion. | | 23091069
 |
Stepwise proteolytic activation of type I procollagen to collagen within the secretory pathway of tendon fibroblasts in situ.
Canty-Laird, EG; Lu, Y; Kadler, KE
The Biochemical journal
441
707-17
2012
Show Abstract
Proteolytic cleavage of procollagen I to collagen I is essential for the formation of collagen fibrils in the extracellular matrix of vertebrate tissues. Procollagen is cleaved by the procollagen N- and C-proteinases, which remove the respective N- and C-propeptides from procollagen. Procollagen processing is initiated within the secretory pathway in tendon fibroblasts, which are adept in assembling an ordered extracellular matrix of collagen fibrils in vivo. It was thought that intracellular processing was restricted to the TGN (trans-Golgi network). In the present study, brefeldin A treatment of tendon explant cultures showed that N-proteinase activity is present in the resulting fused ER (endoplasmic reticulum)-Golgi compartment, but that C-proteinase activity is restricted to the TGN in embryonic chick tendon fibroblasts. In late embryonic and postnatal rat tail and postnatal mouse tail tendon, C-proteinase activity was detected in TGN and pre-TGN compartments. Preventing activation of the procollagen N- and C-proteinases with the furin inhibitor Dec-RVKR-CMK (decanoyl-Arg-Val-Lys-Arg-chloromethylketone) indicated that only a fraction of intracellular procollagen cleavage was mediated by newly activated proteinases. In conclusion, the N-propeptides are removed earlier in the secretory pathway than the C-propeptides. The removal of the C-propeptides in post-Golgi compartments most probably indicates preparation of collagen molecules for fibril formation at the cell-matrix interface. | | 21967573
 |
Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex.
Wang, Y; McNiven, MA
The Journal of cell biology
196
375-85
2012
Show Abstract
Tumor cell migration and the concomitant degradation of extracellular matrix (ECM) are two essential steps in the metastatic process. It is well established that focal adhesions (FAs) play an important role in regulating migration; however, whether these structures contribute to matrix degradation is not clear. In this study, we report that multiple cancer cell lines display degradation of ECM at FA sites that requires the targeted action of MT1-MMP. Importantly, we have found that this MT1-MMP targeting is dependent on an association with a FAK-p130Cas complex situated at FAs and is regulated by Src-mediated phosphorylation of Tyr 573 at the cytoplasmic tail of MT1. Disrupting the FAK-p130Cas-MT1 complex significantly impairs FA-mediated degradation and tumor cell invasion yet does not appear to affect invadopodia formation or function. These findings demonstrate a novel function for FAs and also provide molecular insights into MT1-MMP targeting and function. | | 22291036
 |
VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration.
Williams, BB; Cantrell, VA; Mundell, NA; Bennett, AC; Quick, RE; Jessen, JR
Journal of cell science
125
2141-7
2012
Show Abstract
Planar cell polarity (PCP) describes the polarized orientation of cells within the plane of a tissue. Unlike epithelial PCP, the mechanisms underlying PCP signaling in migrating cells remain undefined. Here, the establishment of PCP must be coordinated with dynamic changes in cell adhesion and extracellular matrix (ECM) organization. During gastrulation, the membrane type-1 matrix metalloproteinase (MT1-MMP or MMP14) is required for PCP and convergence and extension cell movements. We report that the PCP protein Vang-like 2 (VANGL2) regulates the endocytosis and cell-surface availability of MMP14 in manner that is dependent on focal adhesion kinase. We demonstrate that zebrafish trilobite/vangl2 mutant embryos exhibit increased Mmp14 activity and decreased ECM. Furthermore, in vivo knockdown of Mmp14 partially rescues the Vangl2 loss-of-function convergence and extension phenotype. This study identifies a mechanism linking VANGL2 with MMP14 trafficking and suggests that establishment of PCP in migrating gastrula cells requires regulated proteolytic degradation or remodeling of the ECM. Our findings implicate matrix metalloproteinases as downstream effectors of PCP and suggest a broadly applicable mechanism whereby VANGL2 affects diverse morphogenetic processes. | | 22357946
 |
Cancer cell-associated MT1-MMP promotes blood vessel invasion and distant metastasis in triple-negative mammary tumors.
Perentes, JY; Kirkpatrick, ND; Nagano, S; Smith, EY; Shaver, CM; Sgroi, D; Garkavtsev, I; Munn, LL; Jain, RK; Boucher, Y
Cancer research
71
4527-38
2011
Show Abstract
Functional roles for the cancer cell-associated membrane type I matrix metalloproteinase (MT1-MMP) during early steps of the metastatic cascade in primary tumors remain unresolved. In an effort to determine its significance, we determined the in vivo effects of RNAi-mediated downregulation in mammary cancer cells on the migration, blood and lymphatic vessel invasion (LVI), and lymph node and lung metastasis. We also correlated the expression of cancer cell MT1-MMP with blood vessel invasion (BVI) in 102 breast cancer biopsies. MT1-MMP downregulation in cancer cells decreased lung metastasis without affecting primary tumor growth. The inhibition of lung metastasis correlated with reduced cancer cell migration and BVI. Furthermore, cancer cell-expressed MT1-MMP upregulated the expression of MT1-MMP in vascular endothelial cells, but did not affect MT1-MMP expression in lymphatic endothelial cells, LVI, or lymph node metastasis. Of clinical importance, we observed that elevated MT1-MMP expression correlated with BVI in biopsies from triple-negative breast cancers (TNBC), which have a poor prognosis and high incidence of distant metastasis, relative to other breast cancer subtypes. Together, our findings established that MT1-MMP activity in breast tumors is essential for BVI, but not LVI, and that MT1-MMP should be further explored as a predictor and therapeutic target of hematogenous metastasis in TNBC patients. | | 21571860
 |
MT1-MMP plays an important role in an invasive activity of malignant pleural mesothelioma cell.
Doi T, Maniwa Y, Tanaka Y, Tane S, Hashimoto S, Ohno Y, Nishio W, Nishimura Y, Ohbayashi C, Okita Y, Hayashi Y, Yoshimura M.
Experimental and molecular pathology
90
91-6
2011
Show Abstract
Malignant pleural mesothelioma (MPM) has a poor prognosis and is a treatment resistant tumor, which is increasing in frequency throughout the world. The poor prognosis is due to the aggressive local invasiveness rather than distant metastasis. In this study, we established a cell line of malignant mesothelioma from a clinical specimen and assessed the relationship between the expression of MT1-MMP and the invasion ability of that line, as well as the cultured cells of several other lines, using the simple method that we created previously. We established a cell line from a clinical specimen from a patient with malignant mesothelioma. We assessed the invasive activities of MPM cells in an easy-to-prepare double-layered collagen gel hemisphere (DL-CGH) system that enabled us to visualize cell movements during invasion. To assess the role of MT1-MMP in the invasive activity of MPM cells, we knocked down its expression by RNA interference (RNAi). The invasion assay with DL-CGH revealed that a high expression of MT1-MMP in MPM cells was associated with aggressive invasive activity. The RNAi of MT1-MMP indicated that the expression of MT1-MMP might have a crucial role in the invasiveness of MPM cells. The MT1-MMP expression in MPM cells is related to their capacity for locally aggressive spreading into the pleura and the surrounding tissues, and MT1-MMP should be a suitable molecular target for the suppression of the invasiveness of MPM. | | 20969861
 |
KIF5B and KIF3A/KIF3B kinesins drive MT1-MMP surface exposure, CD44 shedding, and extracellular matrix degradation in primary macrophages.
Wiesner, Christiane, et al.
Blood, 116: 1559-69 (2010)
2010
Show Abstract
The matrix metalloproteinase (MMP) MT1-MMP plays pivotal roles in leukocyte physiology such as monocyte diapedesis, dendritic cell migration, and T-cell homing. MT1-MMP is a surface-anchored "master switch" proteinase that cleaves a variety of substrates including extracellular matrix components, matrix receptors, and also other MMPs. However, little is known about the mechanisms enabling intracellular trafficking and exposure of MT1-MMP on the cell surface. We now show that, in primary human macrophages, MT1-MMP-positive vesicles travel bidirectionally along microtubules, in a process regulated by KIF5B and KIF3A/KIF3B kinesins. SiRNA-induced knockdown revealed that transport by KIF5B and KIF3A/KIF3B is crucial for delivery of MT1-MMP to the cell surface and also for surface-associated functions of MT1-MMP, such as shedding of the matrix receptors CD44 and syndecan-1 or degradation of extracellular matrix at podosomes. These data show that kinesin-mediated intracellular transport of MT1-MMP is a pivotal process that allows macrophages to dynamically modify their pericellular environment. These data also identify specific kinesins as potential targets for the early manipulation of MT1-MMP activity in tissues. | | 20505159
 |