438186 Sigma-AldrichLovastatin, Sodium Salt - Calbiochem
Carboxylate form of Lovastatin that is active in whole cells and cell-free assays.
More>> Carboxylate form of Lovastatin that is active in whole cells and cell-free assays. Less<<Synonyms: L-Type Calcium Channel Blocker IV
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₂₄H₃₇O₆ • Na |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 438186-5MG |
|
Plastic ampoule | 5 mg |
|
— |
| Description | |
|---|---|
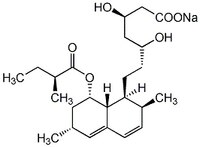
| Overview | Carboxylate form of Lovastatin (Cat. No. 438185) that is active in whole cells and cell-free assays. |
| Catalogue Number | 438186 |
| Brand Family | Calbiochem® |
| Synonyms | L-Type Calcium Channel Blocker IV |
| Product Information | |
|---|---|
| ATP Competitive | N |
| Form | Off-white solid |
| Hill Formula | C₂₄H₃₇O₆ • Na |
| Chemical formula | C₂₄H₃₇O₆ • Na |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ200 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 438186-5MG | 07790788050276 |
Documentation
Lovastatin, Sodium Salt - Calbiochem SDS
| Title |
|---|
Lovastatin, Sodium Salt - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 438186 |
References
| Reference overview |
|---|
| Rao, S., et al. 1999. Proc. Natl. Acad. Sci. USA 96, 7197. Carel, K., et al. 1996. J. Biol. Chem. 271, 30625. McGuire, T.F., et al. 1996. J. Biol. Chem. 271, 27402. Umetani, N., et al. 1996. Biochim. Biophys. Acta 1303, 199. Xu, X.Q., et al. 1996. Arch. Biochem. Biophys. 326, 233. Reusch, J.E.-B., et al. 1995. J. Biol. Chem. 270, 2036. |