Renal Subcapsular Transplantation of PSC-Derived Kidney Organoids Induces Neo-vasculogenesis and Significant Glomerular and Tubular Maturation In Vivo.
van den Berg, CW; Ritsma, L; Avramut, MC; Wiersma, LE; van den Berg, BM; Leuning, DG; Lievers, E; Koning, M; Vanslambrouck, JM; Koster, AJ; Howden, SE; Takasato, M; Little, MH; Rabelink, TJ
Stem Cell Reports
10
751-765
2018
Abstract anzeigen
Human pluripotent stem cell (hPSC)-derived kidney organoids may facilitate disease modeling and the generation of tissue for renal replacement. Long-term application, however, will require transferability between hPSC lines and significant improvements in organ maturation. A key question is whether time or a patent vasculature is required for ongoing morphogenesis. Here, we show that hPSC-derived kidney organoids, derived in fully defined medium conditions and in the absence of any exogenous vascular endothelial growth factor, develop host-derived vascularization. In vivo imaging of organoids under the kidney capsule confirms functional glomerular perfusion as well as connection to pre-existing vascular networks in the organoids. Wide-field electron microscopy demonstrates that transplantation results in formation of a glomerular basement membrane, fenestrated endothelial cells, and podocyte foot processes. Furthermore, compared with non-transplanted organoids, polarization and segmental specialization of tubular epithelium are observed. These data demonstrate that functional vascularization is required for progressive morphogenesis of human kidney organoids. | 29503086
 |
Characterization of induced tissue-specific stem cells from pancreas by a synthetic self-replicative RNA.
Miyagi-Shiohira, C; Nakashima, Y; Kobayashi, N; Saitoh, I; Watanabe, M; Noguchi, H
Sci Rep
8
12341
2018
Abstract anzeigen
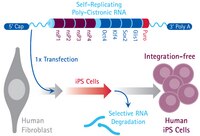
Induced pluripotent stem (iPS) cells have significant implications for overcoming most of the ethical issues associated with embryonic stem (ES) cells. Furthermore, our recent study demonstrated the generation of induced tissue-specific stem (iTS) cells by transient overexpression of the reprogramming factors using a plasmid combined with tissue-specific selection. In this study, we were able to generate RNA-based iTS cells that utilize a single, synthetic, self-replicating VEE-RF RNA replicon expressing four reprogramming factors (OCT4, KLF4, SOX2, and GLIS1). A single VEE-RF RNA transfection into mouse pancreatic tissue resulted in efficient generation of iTS cells from pancreas (iTS-P cells) with genetic markers of endoderm and pancreatic progenitors and differentiation into insulin-producing cells more efficiently than ES cells. Subcutaneous transplantation of iTS-P cells into immunodeficient mice resulted in no teratoma formation. Bisulfite genomic sequencing demonstrated that the promoters of Oct4 and Nanog remained partially methylated in iTS-P cells. We compared the global gene-expression profiles of ES cells, iTS-P cells, and pancreatic islets. Microarray analyses confirmed that the iTS-P cells were similar but not identical to ES cells compared with islets. These data suggest that iTS-P cells are cells that inherit numerous components of epigenetic memory from pancreas cells and acquire self-renewal potential. The generation of iTS cells may have important implications for the clinical application of stem cells. | 30120295
 |
An integration-free, virus-free rhesus macaque induced pluripotent stem cell line (riPSC90) from embryonic fibroblasts.
Sosa, E; Kim, R; Rojas, EJ; Hosohama, L; Hennebold, JD; Orwig, KE; Clark, AT
Stem Cell Res
21
5-8
2016
Abstract anzeigen
The rhesus macaque induced pluripotent stem cell (riPSC) line, UCLAi090-A (riPSC90), was generated from rhesus embryonic fibroblast (REF) cells called REF90. REF90 cells and the riPSC90 line were authenticated by short tandem repeat analysis and had a normal male (42, XY) karyotype. The riPSC90 line expressed markers of self-renewal including OCT4, NANOG, TRA-1-81 and SSEA4, and generated teratomas after transplantation into immunocompromised mice. riPSC90 could be used in parallel with riPSC89, which was derived from REFs cultured from a different rhesus macaque embryo (Sosa et al. 2016). | 28677539
 |













 Ab[184408-ALL].jpg)