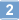569396 Sigma-AldrichInSolution™ Staurosporine, Streptomyces sp.
InSolution™ Staurosporine, Streptomyces sp. CAS 62996-74-1, is provided as a 1 mM (100 µg/214 µl) solution of Staurosporine (Cat. No. 569397) in DMSO
More>> InSolution™ Staurosporine, Streptomyces sp. CAS 62996-74-1, is provided as a 1 mM (100 µg/214 µl) solution of Staurosporine (Cat. No. 569397) in DMSO Less<<Synonyme: MLCK Inhibitor I, PKA Inhibitor II
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 62996-74-1 | C₂₈H₂₆N₄O₃ |
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 569396-100UG |
|
Kst.-Ampulle | 100 μg |
|
— |
| Description | |
|---|---|
| Catalogue Number | 569396 |
| Brand Family | Calbiochem® |
| Synonyms | MLCK Inhibitor I, PKA Inhibitor II |
| Product Information | |
|---|---|
| CAS number | 62996-74-1 |
| ATP Competitive | Y |
| Form | Liquid |
| Formulation | A 1 mM (100 µg/214 µl) solution of Staurosporine (Cat. No. 569397) in DMSO. |
| Hill Formula | C₂₈H₂₆N₄O₃ |
| Chemical formula | C₂₈H₂₆N₄O₃ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications | |
|---|---|
| Application | InSolution™ Staurosporine, Streptomyces sp. CAS 62996-74-1, is provided as a 1 mM (100 µg/214 µl) solution of Staurosporine (Cat. No. 569397) in DMSO |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information | |
|---|---|
| R Phrase | R: 36/38 Irritating to eyes and skin. |
| S Phrase | S: 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 569396-100UG | 07790788051693 |
Documentation
InSolution™ Staurosporine, Streptomyces sp. SDB
| Titel |
|---|
InSolution™ Staurosporine, Streptomyces sp. Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 569396 |
Literatur
| Übersicht |
|---|
| Couldwell, W.T., et al. 1994. FEBS Lett. 345, 43. Nishimura, H. and Simpson, I.A. 1994. Biochem. J. 302, 271. Bruno, S., et al. 1992. Cancer Res. 52, 470. Kiss, Z. and Deli, E. 1992. Biochem. J. 288, 853. Vitale, M.L., et al. 1992. Neuroscience 51, 463. Hoffman, R. and Newland, E.S. 1991. Cancer Chemotherap. Pharmacol. 28, 102. Oka, S., et al. 1986. Agric. Biol. Chem. 50, 2723. |
Literaturstellen
| Titel | |
|---|---|
|
|
| Datenblatt | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|