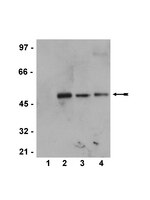
Reactivation of L1 retrotransposon by benzo(a)pyrene involves complex genetic and epigenetic regulation.
Teneng, I; Montoya-Durango, DE; Quertermous, JL; Lacy, ME; Ramos, KS
Epigenetics
6
355-67
2010
Abstract anzeigen
Benzo(a)pyrene (BaP), is an environmental pollutant present in tobacco smoke and a byproduct of fossil fuel combustion which likely contributes to the tumorigenic processes in human cancers including lung and esophageal. Long Interspersed Nuclear Element-1 (LINE-1) or L1 is a mobile element within the mammalian genome that propagates via a "copy-and-paste" mechanism using reverse transcriptase and RNA intermediates. L1 is strongly expressed during early embryogenesis and then silenced as cells initiate differentiation programming. Although the complex transcriptional control mechanisms of L1 are not well understood, L1 reactivation has been described in several human cancers and following exposure of mouse or human cells to BaP. In this study we investigated the molecular mechanisms and epigenetic events that regulate L1 reactivation following BaP exposure. We show that challenge of HeLa cells with BaP induces early enrichment of the transcriptionally-active chromatin markers histone H3 trimethylated at lysine 4 (H3K4Me3) and histone H3 acetylated at lysine 9 (H3K9Ac), and reduces association of DNA methyltransferase-1 (DNMT1) with the L1 promoter. These changes are followed by proteasome-dependent decreases in cellular DNMT1 expression and sustained reduction of cytosine methylation within the L1 promoter CpG island. Pharmacological inhibition of the proteasome signaling pathway with the inhibitor MG132 blocks degradation of DNMT1 and alters BaP-mediated histone epigenetic modifications. We conclude that genetic reactivation of L1 by BaP involves an ordered cascade of epigenetic events that begin with nucleosomal histone modifications and is completed with alterations in DNMT1 recruitment to the L1 promoter and reduced DNA methylation of CpG islands. | 21150308
 |
The 5-HT 2A serotonin receptor enhances cell viability, affects cell cycle progression and activates MEK-ERK1/2 and JAK2-STAT3 signalling pathways in human choriocarcinoma cell lines.
Oufkir T, Arseneault M, Sanderson JT, Vaillancourt C
Placenta
31
439-47. Epub 2010 Mar 25.
2009
Abstract anzeigen
Previous results from our group have demonstrated the expression of the 5-HT(2A) receptor and a mitogenic effect of serotonin in human trophoblast. The objectives of the present study were to investigate the role of the 5-HT(2A) receptor in trophoblast cells and to determine the signalling pathways activated by this receptor. We investigated the effect of (+/-)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI), a selective 5-HT(2A) agonist, on cell cycle progression and cell viability in BeWo and JEG-3 cells. We also investigated, by co-immunoprecipitation and western blot analysis, the involvement of the MEK-ERK1/2 and JAK2-STAT3 signalling pathways following activation of the placental 5-HT(2A) receptor. Our results showed a concentration-dependent increase of cell viability by DOI, which was reversed by ketanserin, a selective 5-HT(2A) receptor antagonist. Furthermore, activation of the 5-HT(2A) receptor by DOI increased cell entry into the G2/M and S phase (DNA synthesis) in BeWo and JEG-3 cells, respectively. In addition, stimulation of BeWo and JEG-3 cells by DOI activated both the MEK-ERK1/2 and the JAK2-STAT3 signalling pathways. This study demonstrated that the 5-HT(2A) receptor increases cell viability and affects cell cycle progression in human trophoblast cell lines as well as activates the MEK-ERK1/2 and JAK2-STAT3 intracellular signalling pathways, which are related to survival, differentiation, migration and invasion. These findings indicate that serotonin through the activation of the 5-HT(2A) receptor is a key regulator of placentation and may play a role in the pathophysiology of certain pregnancy disorders associated with alterations in placental development, such as preeclampsia, gestational diabetes and preterm birth. | 20338635
 |
AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade.
F Donelson Smith,Lorene K Langeberg,Cristina Cellurale,Tony Pawson,Deborah K Morrison,Roger J Davis,John D Scott
Nature cell biology
12
2009
Abstract anzeigen
Mitogen-activated protein kinase (MAPK) cascades propagate a variety of cellular activities. Processive relay of signals through RAF-MEK-ERK modulates cell growth and proliferation. Signalling through this ERK cascade is frequently amplified in cancers, and drugs such as sorafenib (which is prescribed to treat renal and hepatic carcinomas) and PLX4720 (which targets melanomas) inhibit RAF kinases. Natural factors that influence ERK1/2 signalling include the second messenger cyclic AMP. However, the mechanisms underlying this cascade have been difficult to elucidate. We demonstrate that the A-kinase-anchoring protein AKAP-Lbc and the scaffolding protein kinase suppressor of Ras (KSR-1) form the core of a signalling network that efficiently relay signals from RAF, through MEK, and on to ERK1/2. AKAP-Lbc functions as an enhancer of ERK signalling by securing RAF in the vicinity of MEK1 and synchronizing protein kinase A (PKA)-mediated phosphorylation of Ser 838 on KSR-1. This offers mechanistic insight into cAMP-responsive control of ERK signalling events. Volltextartikel | 21102438
 |
Negative regulation of MAPKK by phosphorylation of a conserved serine residue equivalent to Ser212 of MEK1.
Gopalbhai, Kailesh, et al.
J. Biol. Chem., 278: 8118-25 (2003)
2003
Abstract anzeigen
The MAPKKs MEK1 and MEK2 are activated by phosphorylation, but little is known about how these enzymes are inactivated. Here, we show that MEK1 is phosphorylated in vivo at Ser(212), a residue conserved among all MAPKK family members. Mutation of Ser(212) to alanine enhanced the basal activity of MEK1, whereas the phosphomimetic aspartate mutation completely suppressed the activation of both wild-type MEK1 and the constitutively activated MEK1(S218D/S222D) mutant. Phosphorylation of Ser(212) did not interfere with activating phosphorylation of MEK1 at Ser(218)/Ser(222) or with binding to ERK2 substrate. Importantly, mimicking phosphorylation of the equivalent Ser(212) residue of the yeast MAPKKs Pbs2p and Ste7p similarly abrogated their biological function. Our findings suggest that Ser(212) phosphorylation represents an evolutionarily conserved mechanism involved in the negative regulation of MAPKKs. | 12506122
 |
Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2.
Hodge, C, et al.
J. Biol. Chem., 273: 31327-36 (1998)
1998
Abstract anzeigen
Growth hormone (GH), a major regulator of normal body growth and metabolism, regulates cellular gene expression. The transcription factors Elk-1 and Serum Response Factor are necessary for GH-stimulated transcription of c-fos through the Serum Response Element (SRE). GH stimulates the serine phosphorylation of Elk-1, thereby enabling Elk-1 to mediate transcriptional activation. The contribution of the Ras/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway to Elk-1-mediated transcriptional activation of the c-fos SRE in response to GH was examined. The MEK inhibitor PD098059 attenuated GH-induced expression of the endogenous SRE-regulated genes c-fos, egr-1, and junB as well as transcriptional activation mediated by the c-fos promoter. The MEK inhibitor blocked GH-stimulated activation of MEK, phosphorylation of ERK1/ERK2, and MAP kinase activity in 3T3-F442A cells. Blocking MEK activation prevented GH-induced phosphorylation of Elk-1, as well as the ability of Elk-1 to mediate transcriptional activation in response to GH. Overexpression of dominant-negative Ras or the ERK-specific phosphatase, mitogen-activated protein kinase phosphatase-1, blocked the Ras/MEK/ERK pathway and abrogated GH-induced phosphorylation of Elk-1. GH failed to stimulate phosphorylation or activation of Jun N-terminal kinase under the conditions used. GH slightly increased p38-mediated mitogen-activated protein kinase-activated protein (MAPKAP) kinase-2 activity, but the p38 inhibitor SB203580 did not attenuate GH-promoted Elk-1 phosphorylation. Wortmannin, which inhibited GH-induced ERK phosphorylation, also attenuated transcriptional activation of c-fos by GH. Taken together, these data suggest that GH-dependent activation of the Ras/MEK/ERK pathway and subsequent serine phosphorylation of Elk-1 contribute to GH-stimulated c-fos expression through the SRE. | 9813041
 |