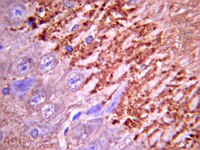
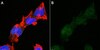
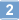A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions.
Zahavi, EE; Ionescu, A; Gluska, S; Gradus, T; Ben-Yaakov, K; Perlson, E
Journal of cell science
128
1241-52
2015
Abstract anzeigen
Bidirectional molecular communication between the motoneuron and the muscle is vital for neuromuscular junction (NMJ) formation and maintenance. The molecular mechanisms underlying such communication are of keen interest and could provide new targets for intervention in motoneuron disease. Here, we developed a microfluidic platform with motoneuron cell bodies on one side and muscle cells on the other, connected by motor axons extending through microgrooves to form functional NMJs. Using this system, we were able to differentiate between the proximal and distal effects of oxidative stress and glial-derived neurotrophic factor (GDNF), demonstrating a dying-back degeneration and retrograde transmission of pro-survival signaling, respectively. Furthermore, we show that GDNF acts differently on motoneuron axons versus soma, promoting axonal growth and innervation only when applied locally to axons. Finally, we track for the first time the retrograde transport of secreted GDNF from muscle to neuron. Thus, our data suggests spatially distinct effects of GDNF--facilitating growth and muscle innervation at axon terminals and survival pathways in the soma. | Immunocytochemistry | | 25632161
 |
Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model.
Sidhu, H; Dansie, LE; Hickmott, PW; Ethell, DW; Ethell, IM
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
9867-79
2014
Abstract anzeigen
Fmr1 knock-out (ko) mice display key features of fragile X syndrome (FXS), including delayed dendritic spine maturation and FXS-associated behaviors, such as poor socialization, obsessive-compulsive behavior, and hyperactivity. Here we provide conclusive evidence that matrix metalloproteinase-9 (MMP-9) is necessary to the development of FXS-associated defects in Fmr1 ko mice. Genetic disruption of Mmp-9 rescued key aspects of Fmr1 deficiency, including dendritic spine abnormalities, abnormal mGluR5-dependent LTD, as well as aberrant behaviors in open field and social novelty tests. Remarkably, MMP-9 deficiency also corrected non-neural features of Fmr1 deficiency-specifically macroorchidism-indicating that MMP-9 dysregulation contributes to FXS-associated abnormalities outside the CNS. Further, MMP-9 deficiency suppressed elevations of Akt, mammalian target of rapamycin, and eukaryotic translation initiation factor 4E phosphorylation seen in Fmr1 ko mice, which are also associated with other autistic spectrum disorders. These findings establish that MMP-9 is critical to the mechanisms responsible for neural and non-neural aspects of the FXS phenotype. | Immunofluorescence | | 25057190
 |
Synaptic function is modulated by LRRK2 and glutamate release is increased in cortical neurons of G2019S LRRK2 knock-in mice.
Beccano-Kelly, DA; Kuhlmann, N; Tatarnikov, I; Volta, M; Munsie, LN; Chou, P; Cao, LP; Han, H; Tapia, L; Farrer, MJ; Milnerwood, AJ
Frontiers in cellular neuroscience
8
301
2014
Abstract anzeigen
Mutations in Leucine-Rich Repeat Kinase-2 (LRRK2) result in familial Parkinson's disease and the G2019S mutation alone accounts for up to 30% in some ethnicities. Despite this, the function of LRRK2 is largely undetermined although evidence suggests roles in phosphorylation, protein interactions, autophagy and endocytosis. Emerging reports link loss of LRRK2 to altered synaptic transmission, but the effects of the G2019S mutation upon synaptic release in mammalian neurons are unknown. To assess wild type and mutant LRRK2 in established neuronal networks, we conducted immunocytochemical, electrophysiological and biochemical characterization of greater than 3 week old cortical cultures of LRRK2 knock-out, wild-type overexpressing and G2019S knock-in mice. Synaptic release and synapse numbers were grossly normal in LRRK2 knock-out cells, but discretely reduced glutamatergic activity and reduced synaptic protein levels were observed. Conversely, synapse density was modestly but significantly increased in wild-type LRRK2 overexpressing cultures although event frequency was not. In knock-in cultures, glutamate release was markedly elevated, in the absence of any change to synapse density, indicating that physiological levels of G2019S LRRK2 elevate probability of release. Several pre-synaptic regulatory proteins shown by others to interact with LRRK2 were expressed at normal levels in knock-in cultures; however, synapsin 1 phosphorylation was significantly reduced. Thus, perturbations to the pre-synaptic release machinery and elevated synaptic transmission are early neuronal effects of LRRK2 G2019S. Furthermore, the comparison of knock-in and overexpressing cultures suggests that one copy of the G2019S mutation has a more pronounced effect than an ~3-fold increase in LRRK2 protein. Mutant-induced increases in transmission may convey additional stressors to neuronal physiology that may eventually contribute to the pathogenesis of Parkinson's disease. | Immunofluorescence | | 25309331
 |
Structural basis for extracellular cis and trans RPTPσ signal competition in synaptogenesis.
Coles, CH; Mitakidis, N; Zhang, P; Elegheert, J; Lu, W; Stoker, AW; Nakagawa, T; Craig, AM; Jones, EY; Aricescu, AR
Nature communications
5
5209
2014
Abstract anzeigen
Receptor protein tyrosine phosphatase sigma (RPTPσ) regulates neuronal extension and acts as a presynaptic nexus for multiple protein and proteoglycan interactions during synaptogenesis. Unknown mechanisms govern the shift in RPTPσ function, from outgrowth promotion to synaptic organization. Here, we report crystallographic, electron microscopic and small-angle X-ray scattering analyses, which reveal sufficient inter-domain flexibility in the RPTPσ extracellular region for interaction with both cis (same cell) and trans (opposite cell) ligands. Crystal structures of RPTPσ bound to its postsynaptic ligand TrkC detail an interaction surface partially overlapping the glycosaminoglycan-binding site. Accordingly, heparan sulphate and heparin oligomers compete with TrkC for RPTPσ binding in vitro and disrupt TrkC-dependent synaptic differentiation in neuronal co-culture assays. We propose that transient RPTPσ ectodomain emergence from the presynaptic proteoglycan layer allows capture by TrkC to form a trans-synaptic complex, the consequent reduction in RPTPσ flexibility potentiating interactions with additional ligands to orchestrate excitatory synapse formation. | | | 25385546
 |
Live imaging of endogenous PSD-95 using ENABLED: a conditional strategy to fluorescently label endogenous proteins.
Fortin, DA; Tillo, SE; Yang, G; Rah, JC; Melander, JB; Bai, S; Soler-Cedeño, O; Qin, M; Zemelman, BV; Guo, C; Mao, T; Zhong, H
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
16698-712
2014
Abstract anzeigen
Stoichiometric labeling of endogenous synaptic proteins for high-contrast live-cell imaging in brain tissue remains challenging. Here, we describe a conditional mouse genetic strategy termed endogenous labeling via exon duplication (ENABLED), which can be used to fluorescently label endogenous proteins with near ideal properties in all neurons, a sparse subset of neurons, or specific neuronal subtypes. We used this method to label the postsynaptic density protein PSD-95 with mVenus without overexpression side effects. We demonstrated that mVenus-tagged PSD-95 is functionally equivalent to wild-type PSD-95 and that PSD-95 is present in nearly all dendritic spines in CA1 neurons. Within spines, while PSD-95 exhibited low mobility under basal conditions, its levels could be regulated by chronic changes in neuronal activity. Notably, labeled PSD-95 also allowed us to visualize and unambiguously examine otherwise-unidentifiable excitatory shaft synapses in aspiny neurons, such as parvalbumin-positive interneurons and dopaminergic neurons. Our results demonstrate that the ENABLED strategy provides a valuable new approach to study the dynamics of endogenous synaptic proteins in vivo. | Western Blotting | | 25505322
 |
Distribution of Na,K-ATPase α subunits in rat vestibular sensory epithelia.
Schuth, O; McLean, WJ; Eatock, RA; Pyott, SJ
Journal of the Association for Research in Otolaryngology : JARO
15
739-54
2014
Abstract anzeigen
The afferent encoding of vestibular stimuli depends on molecular mechanisms that regulate membrane potential, concentration gradients, and ion and neurotransmitter clearance at both afferent and efferent relays. In many cell types, the Na,K-ATPase (NKA) is essential for establishing hyperpolarized membrane potentials and mediating both primary and secondary active transport required for ion and neurotransmitter clearance. In vestibular sensory epithelia, a calyx nerve ending envelopes each type I hair cell, isolating it over most of its surface from support cells and posing special challenges for ion and neurotransmitter clearance. We used immunofluorescence and high-resolution confocal microscopy to examine the cellular and subcellular patterns of NKAα subunit expression within the sensory epithelia of semicircular canals as well as an otolith organ (the utricle). Results were similar for both kinds of vestibular organ. The neuronal NKAα3 subunit was detected in all afferent endings-both the calyx afferent endings on type I hair cells and bouton afferent endings on type II hair cells-but was not detected in efferent terminals. In contrast to previous results in the cochlea, the NKAα1 subunit was detected in hair cells (both type I and type II) but not in supporting cells. The expression of distinct NKAα subunits by vestibular hair cells and their afferent endings may be needed to support and shape the high rates of glutamatergic neurotransmission and spike initiation at the unusual type I-calyx synapse. | Immunohistochemistry | Rat | 25091536
 |
Linker mutations reveal the complexity of synaptotagmin 1 action during synaptic transmission.
Liu, H; Bai, H; Xue, R; Takahashi, H; Edwardson, JM; Chapman, ER
Nature neuroscience
17
670-7
2014
Abstract anzeigen
The Ca(2+) sensor for rapid synaptic vesicle exocytosis, synaptotagmin 1 (syt), is largely composed of two Ca(2+)-sensing C2 domains, C2A and C2B. We investigated the apparent synergy between the tandem C2 domains by altering the length and rigidity of the linker that connects them. The behavior of the linker mutants revealed a correlation between the ability of the C2 domains to penetrate membranes in response to Ca(2+) and to drive evoked neurotransmitter release in cultured mouse neurons, uncovering a step in excitation-secretion coupling. Using atomic force microscopy, we found that the synergy between these C2 domains involved intra-molecular interactions between them. Thus, syt function is markedly affected by changes in the physical nature of the linker that connects its tandem C2 domains. Moreover, the linker mutations uncoupled syt-mediated regulation of evoked and spontaneous release, revealing that syt also acts as a fusion clamp before the Ca(2+) trigger. | | | 24657966
 |
Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects.
Smalheiser, NR; Lugli, G; Zhang, H; Rizavi, H; Cook, EH; Dwivedi, Y
PloS one
9
e86469
2014
Abstract anzeigen
Because of the role played by miRNAs in post-transcriptional regulation of an array of genes, their impact in neuropsychiatric disease pathophysiology has increasingly been evident. In the present study, we assessed microRNA expression in prefrontal cortex (Brodmann area 10) of a well-characterized cohort of major depressed, bipolar, and schizophrenia subjects (obtained from Stanley Neuropathology Consortium; n = 15 in each group), using high throughput RT-PCR plates. Discrete miRNA alterations were observed in all disorders, as well as in suicide subjects (pooled across diagnostic categories) compared to all non-suicide subjects. The changes in the schizophrenia group were partially similar to those in the bipolar group, but distinct from changes in depression and suicide. Intriguingly, those miRNAs which were down-regulated in the schizophrenia group tended to be synaptically enriched, whereas up-regulated miRNAs tended not to be. To follow this up, we purified synaptosomes from pooled samples of the schizophrenia vs. control groups and subjected them to Illumina deep sequencing. There was a significant loss of small RNA expression in schizophrenia synaptosomes only for certain sequence lengths within the miRNA range. Moreover, 73 miRNAs were significantly down-regulated whereas only one was up-regulated. Strikingly, across all expressed miRNAs in synaptosomes, there was a significant inverse correlation between the fold-change of a given miRNA seen in schizophrenia and its synaptic enrichment ratio observed in controls. Thus, synaptic miRNAs tended to be down-regulated in schizophrenia, and the more highly synaptically enriched miRNAs tended to show greater down-regulation. These findings point to some deficit in miRNA biogenesis, transport, processing or turnover in schizophrenia that is selective for the synaptic compartment. A novel class of ncRNA-derived small RNAs, shown to be strongly induced during an early phase of learning in mouse, is also expressed in man, and at least one representative (SNORD85) was strongly down-regulated in schizophrenia synaptosomes. | | | 24475125
 |
Microglia actively regulate the number of functional synapses.
Ji, K; Akgul, G; Wollmuth, LP; Tsirka, SE
PloS one
8
e56293
2013
Abstract anzeigen
Microglia are the immunocompetent cells of the central nervous system. In the physiological setting, their highly motile processes continually survey the local brain parenchyma and transiently contact synaptic elements. Although recent work has shown that the interaction of microglia with synapses contributes to synaptic remodeling during development, the role of microglia in synaptic physiology is just starting to get explored. To assess this question, we employed an electrophysiological approach using two methods to manipulate microglia in culture: organotypic hippocampal brain slices in which microglia were depleted using clodronate liposomes, and cultured hippocampal neurons to which microglia were added. We show here that the frequency of excitatory postsynaptic current increases in microglia-depleted brain slices, consistent with a higher synaptic density, and that this enhancement ensures from the loss of microglia since it is reversed when the microglia are replenished. Conversely, the addition of microglia to neuronal cultures decreases synaptic activity and reduces the density of synapses, spine numbers, surface expression of AMPA receptor (GluA1), and levels of synaptic adhesion molecules. Taken together, our findings demonstrate that non-activated microglia acutely modulate synaptic activity by regulating the number of functional synapses in the central nervous system. | | | 23393609
 |
Dynein interacts with the neural cell adhesion molecule (NCAM180) to tether dynamic microtubules and maintain synaptic density in cortical neurons.
Perlson, E; Hendricks, AG; Lazarus, JE; Ben-Yaakov, K; Gradus, T; Tokito, M; Holzbaur, EL
The Journal of biological chemistry
288
27812-24
2013
Abstract anzeigen
Cytoplasmic dynein is well characterized as an organelle motor, but dynein also acts to tether and stabilize dynamic microtubule plus-ends in vitro. Here we identify a novel and direct interaction between dynein and the 180-kDa isoform of the neural cell adhesion molecule (NCAM). Optical trapping experiments indicate that dynein bound to beads via the NCAM180 interaction domain can tether projecting microtubule plus-ends. Live cell assays indicate that the NCAM180-dependent recruitment of dynein to the cortex leads to the selective stabilization of microtubules projecting to NCAM180 patches at the cell periphery. The dynein-NCAM180 interaction also enhances cell-cell adhesion in heterologous cell assays. Dynein and NCAM180 co-precipitate from mouse brain extract and from synaptosomal fractions, consistent with an endogenous interaction in neurons. Thus, we examined microtubule dynamics and synaptic density in primary cortical neurons. We find that depletion of NCAM, inhibition of the dynein-NCAM180 interaction, or dampening of microtubule dynamics with low dose nocodazole all result in significantly decreased in synaptic density. Based on these observations, we propose a working model for the role of dynein at the synapse, in which the anchoring of the motor to the cortex via binding to an adhesion molecule mediates the tethering of dynamic microtubule plus-ends to potentiate synaptic stabilization. | | | 23960070
 |