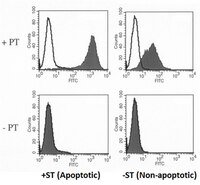
Anti-phospholipid antibodies (aPL) and apoptosis: prothrombin-dependent aPL as a paradigm for phospholipid-dependent interactions with apoptotic cells.
Rauch, J; D'Agnillo, P; Subang, R; Levine, JS
Thrombosis research
114
371-82
2004
Abstract anzeigen
The natural targets of anti-phospholipid antibodies (aPL) and the stimuli that induce them remain unknown. Apoptotic cells have been proposed as both potential targets and immunogens for anti-phospholipid antibodies. Demonstration of selective recognition by anti-phospholipid antibodies provides support for apoptotic cells as antigenic targets. Here, we summarize data showing that prothrombin (PT) binds to apoptotic, but not viable, cells, and that apoptotic-cell bound prothrombin provides a target for human polyclonal and murine monoclonal lupus anticoagulant (LA) antibodies. We discuss findings for two monoclonal lupus anticoagulant antibodies that have high (antibody 29J3-62) or low (antibody 29I4-24) affinity, respectively, for soluble prothrombin. Despite their very different affinities for soluble prothrombin, both monoclonal antibodies reacted similarly with prothrombin bound to phospholipid or apoptotic cells. Furthermore, both antibodies enhanced the binding of prothrombin to apoptotic cells. We propose that the recognition of apoptotic cells by these prothrombin-dependent monoclonal antibodies provides a paradigm for other anti-phospholipid autoantibodies. 29I4-24 is prototypical of phospholipid-dependent anti-phospholipid antibodies, while 29J3-62 represents a prototype for phospholipid-independent anti-phospholipid antibodies. Proteins such as prothrombin and beta2-glycoprotein I (beta2GPI) bind to apoptotic cells, thereby enhancing the recognition of apoptotic cells by anti-phospholipid antibodies. Furthermore, anti-phospholipid antibodies potentiate the interaction of these proteins with apoptotic cells. While it is unclear whether apoptotic cells are the inducing stimuli in patients with anti-phospholipid antibodies or even whether anti-phospholipid antibodies interact with apoptotic cells in vivo, it is nonetheless clear that anti-phospholipid antibodies have the potential to affect both the procoagulant activity and the uptake and clearance of apoptotic cells. | 15507267
 |
Prothrombin binds to the surface of apoptotic, but not viable, cells and serves as a target of lupus anticoagulant autoantibodies.
D'Agnillo, P; Levine, JS; Subang, R; Rauch, J
Journal of immunology (Baltimore, Md. : 1950)
170
3408-22
2003
Abstract anzeigen
Anti-phospholipid Ab (aPL) are a heterogeneous group of autoantibodies directed against various combinations of phospholipids (PL) and PL-binding proteins. Lupus anticoagulant (LA) Ab, a subset of aPL, exhibit anticoagulant properties in vitro, but are procoagulant in vivo. Most LA Ab are specific for either beta(2)-glycoprotein I (beta(2)GPI) or prothrombin (PT), two PL-binding proteins. We have previously shown that beta(2)GPI and beta(2)GPI-dependent aPL bind specifically to apoptotic, but not viable, thymocytes. In this study, we demonstrate that PT, like beta(2)GPI, binds selectively to the surface of apoptotic, but not viable, Jurkat cells. Furthermore, PT supports the binding of systemic lupus erythematosus-derived polyclonal and murine monoclonal LA Ab to apoptotic cells. Two LA mAb, which differed dramatically in their relative affinities for PT, were studied. Although one mAb (29J3-62) had a high affinity for PT alone, the other (29I4-24) showed minimal reactivity with PT alone and required PL for elevated binding. Monovalent fragments of 29I4-24 reacted with PL-bound PT with high affinity, suggesting that this mAb recognizes a PL-dependent epitope. Despite these differences, PT-dependent binding of both mAb to apoptotic cells was 30-fold greater than that to viable cells. Moreover, binding of PT to apoptotic cells was, itself, increased in the presence of bivalent, but not monovalent, forms of either mAb. In summary, our data demonstrate the following: 1) specific binding of PT to apoptotic cells, an effect enhanced by PT-dependent LA Ab; 2) heterogeneity of PT-dependent LA Ab; and 3) potential pathogenicity of Ab of either low or high affinity for PT. | 12626602
 |