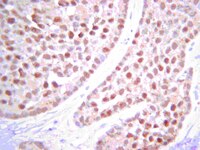
PTEN signaling is required for the maintenance of spermatogonial stem cells in mouse, by regulating the expressions of PLZF and UTF1.
Zhou, W; Shao, H; Zhang, D; Dong, J; Cheng, W; Wang, L; Teng, Y; Yu, Z
Cell & bioscience
5
42
2015
Abstract anzeigen
Pten plays a crucial role in the stem cell maintenance in a few organs. Pten defect also causes the premature oocytes and ovary aging. We and other groups have found that the phosphatidylinositol-3-OH kinase (PI3K)-Akt signaling regulates the proliferation and differentiation of spermatogonial stem cells (SSCs). PTEN functions as a negative regulator of the PI3K pathway. Thus, we thought that the fate of SSCs might be controlled by Pten.We report that promyelocytic leukaemia zinc finger (PLZF) and undifferentiated embryonic cell transcription factor 1 (UTF1), both of which are germ cell-specific transcriptional factors, are regulated by Pten. Conditional deletion of Pten leads to reduction in PLZF expression but induction of UTF1, which is associated with SSCs depletion and infertility in males with age.Our data demonstrate that Pten is required for the long-term maintenance of SSCs and precise regulation of spermatogenesis in mouse. The finding of a Pten-regulated GFRα1(+)/PLZF(-)/UTF1(+) progenitor population provides a new insight into the precise mechanisms controlling SSC fate. | | 26221533
 |
Role of DNA methylation and epigenetic silencing of HAND2 in endometrial cancer development.
Jones, A; Teschendorff, AE; Li, Q; Hayward, JD; Kannan, A; Mould, T; West, J; Zikan, M; Cibula, D; Fiegl, H; Lee, SH; Wik, E; Hadwin, R; Arora, R; Lemech, C; Turunen, H; Pakarinen, P; Jacobs, IJ; Salvesen, HB; Bagchi, MK; Bagchi, IC; Widschwendter, M
PLoS medicine
10
e1001551
2013
Abstract anzeigen
Endometrial cancer incidence is continuing to rise in the wake of the current ageing and obesity epidemics. Much of the risk for endometrial cancer development is influenced by the environment and lifestyle. Accumulating evidence suggests that the epigenome serves as the interface between the genome and the environment and that hypermethylation of stem cell polycomb group target genes is an epigenetic hallmark of cancer. The objective of this study was to determine the functional role of epigenetic factors in endometrial cancer development.Epigenome-wide methylation analysis of greater than 27,000 CpG sites in endometrial cancer tissue samples (n = 64) and control samples (n = 23) revealed that HAND2 (a gene encoding a transcription factor expressed in the endometrial stroma) is one of the most commonly hypermethylated and silenced genes in endometrial cancer. A novel integrative epigenome-transcriptome-interactome analysis further revealed that HAND2 is the hub of the most highly ranked differential methylation hotspot in endometrial cancer. These findings were validated using candidate gene methylation analysis in multiple clinical sample sets of tissue samples from a total of 272 additional women. Increased HAND2 methylation was a feature of premalignant endometrial lesions and was seen to parallel a decrease in RNA and protein levels. Furthermore, women with high endometrial HAND2 methylation in their premalignant lesions were less likely to respond to progesterone treatment. HAND2 methylation analysis of endometrial secretions collected using high vaginal swabs taken from women with postmenopausal bleeding specifically identified those patients with early stage endometrial cancer with both high sensitivity and high specificity (receiver operating characteristics area under the curve = 0.91 for stage 1A and 0.97 for higher than stage 1A). Finally, mice harbouring a Hand2 knock-out specifically in their endometrium were shown to develop precancerous endometrial lesions with increasing age, and these lesions also demonstrated a lack of PTEN expression.HAND2 methylation is a common and crucial molecular alteration in endometrial cancer that could potentially be employed as a biomarker for early detection of endometrial cancer and as a predictor of treatment response. The true clinical utility of HAND2 DNA methylation, however, requires further validation in prospective studies. Please see later in the article for the Editors' Summary. | | 24265601
 |
Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior.
Anastasio, NC; Gilbertson, SR; Bubar, MJ; Agarkov, A; Stutz, SJ; Jeng, Y; Bremer, NM; Smith, TD; Fox, RG; Swinford, SE; Seitz, PK; Charendoff, MN; Craft, JW; Laezza, FM; Watson, CS; Briggs, JM; Cunningham, KA
The Journal of neuroscience : the official journal of the Society for Neuroscience
33
1615-30
2013
Abstract anzeigen
Serotonin (5-hydroxytryptamine; 5-HT) signaling through the 5-HT(2C) receptor (5-HT(2C)R) is essential in normal physiology, whereas aberrant 5-HT(2C)R function is thought to contribute to the pathogenesis of multiple neural disorders. The 5-HT(2C)R interacts with specific protein partners, but the impact of such interactions on 5-HT(2C)R function is poorly understood. Here, we report convergent cellular and behavioral data that the interaction between the 5-HT(2C)R and protein phosphatase and tensin homolog (PTEN) serves as a regulatory mechanism to control 5-HT(2C)R-mediated biology but not that of the closely homologous 5-HT(2A)R. A peptide derived from the third intracellular loop of the human 5-HT(2C)R [3L4F (third loop, fourth fragment)] disrupted the association, allosterically augmented 5-HT(2C)R-mediated signaling in live cells, and acted as a positive allosteric modulator in rats in vivo. We identified the critical residues within an 8 aa fragment of the 3L4F peptide that maintained efficacy (within the picomolar range) in live cells similar to that of the 3L4F peptide. Last, molecular modeling identified key structural features and potential interaction sites of the active 3L4F peptides against PTEN. These compelling data demonstrate the specificity and importance of this protein assembly in cellular events and behaviors mediated by 5-HT(2C)R signaling and provide a chemical guidepost to the future development of drug-like peptide or small-molecule inhibitors as neuroprobes to study 5-HT(2C)R allostery and therapeutics for 5-HT(2C)R-mediated disorders. | | 23345234
 |
BRCAness profile of sporadic ovarian cancer predicts disease recurrence.
Wysham, WZ; Mhawech-Fauceglia, P; Li, H; Hays, L; Syriac, S; Skrepnik, T; Wright, J; Pande, N; Hoatlin, M; Pejovic, T
PloS one
7
e30042
2011
Abstract anzeigen
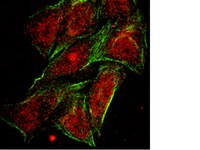
The consequences of defective homologous recombination (HR) are not understood in sporadic ovarian cancer, nor have the potential role of HR proteins other than BRCA1 and BRCA2 been clearly defined. However, it is clear that defects in HR and other DNA repair pathways are important to the effectiveness of current therapies. We hypothesize that a subset of sporadic ovarian carcinomas may harbor anomalies in HR pathways, and that a BRCAness profile (defects in HR or other DNA repair pathways) could influence response rate and survival after treatment with platinum drugs. Clinical availability of a BRCAness profile in patients and/or tumors should improve treatment outcomes.To define the BRCAness profile of sporadic ovarian carcinoma and determine whether BRCA1, PARP, FANCD2, PTEN, H2AX, ATM, and P53 protein expression correlates with response to treatment, disease recurrence, and recurrence-free survival.Protein microarray analysis of ovarian cancer tissue was used to determine protein expression levels for defined DNA repair proteins. Correlation with clinical and pathologic parameters in 186 patients with advanced stage III-IV and grade 3 ovarian cancer was analyzed using Chi square, Kaplan-Meier method, Cox proportional hazard model, and cumulative incidence function.High PARP, FANCD2 and BRCA1 expressions were significantly correlated with each other; however, elevated p53 expression was associated only with high PARP and FANCD2. Of all patients, 9% recurred within the first year. Among early recurring patients, 41% had high levels of PARP, FANCD2 and P53, compared to 19.5% of patients without early recurrence (p = 0.04). Women with high levels of PARP, FANCD2 and/or P53 had first year cumulative cancer incidence of 17% compared with 7% for the other groups (P = 0.03).Patients with concomitantly high levels of PARP, FANCD2 and P53 protein expression are at increased risk of early ovarian cancer recurrence and platinum resistance. | Immunocytochemistry | 22253870
 |
Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity.
Baker, DJ; Jin, F; Jeganathan, KB; van Deursen, JM
Cancer cell
16
475-86
2009
Abstract anzeigen
Genetic alterations that promote chromosome missegregation have been proposed to drive tumorigenesis through loss of whole chromosomes containing key tumor suppressor genes. To test this unproven idea, we bred Bub1 mutant mice that inaccurately segregate their chromosomes onto p53(+/-), Apc(Min/+), Rb(+/-), or Pten(+/-) backgrounds. Bub1 insufficiency predisposed p53(+/-) mice to thymic lymphomas and Apc(Min/+) mice to colonic tumors. These tumors consistently lacked the nonmutated tumor suppressor allele but had gained a copy of the mutant allele. In contrast, Bub1 insufficiency had no impact on tumorigenesis in Rb(+/-) mice and inhibited prostatic intraepithelial neoplasia formation in Pten(+/-) mice. Thus, Bub1 insufficiency can drive tumor formation through tumor suppressor gene loss of heterozygosity, but only in restricted genetic and cellular contexts. | Immunohistochemistry | 19962666
 |