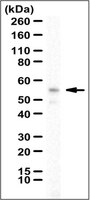
Activation of a PGC-1-related coactivator (PRC)-dependent inflammatory stress program linked to apoptosis and premature senescence.
Gleyzer, N; Scarpulla, RC
The Journal of biological chemistry
288
8004-15
2013
Abstract anzeigen
PGC-1-related coactivator (PRC), a growth-regulated member of the PGC-1 coactivator family, contributes to the expression of the mitochondrial respiratory apparatus. PRC also orchestrates a robust response to metabolic stress by promoting the expression of multiple genes specifying inflammation, proliferation, and metabolic reprogramming. Here, we demonstrate that this PRC-dependent stress program is activated during apoptosis and senescence, two major protective mechanisms against cellular dysfunction. Both PRC and its targets (IL1α, SPRR2D, and SPRR2F) were rapidly induced by menadione, an agent that promotes apoptosis through the generation of intracellular oxidants. Menadione-induced apoptosis and the PRC stress program were blocked by the antioxidant N-acetylcysteine. The PRC stress response was also activated by the topoisomerase I inhibitor 7-ethyl-10-hydroxycamptothecin (SN-38), an inducer of premature senescence in tumor cells. Cells treated with SN-38 displayed morphological characteristics of senescence and express senescence-associated β-galactosidase activity. In contrast to menadione, the SN-38 induction of the PRC program occurred over an extended time course and was antioxidant-insensitive. The potential adaptive function of the PRC stress response was investigated by treating cells with meclizine, a drug that promotes glycolytic energy metabolism and has been linked to cardio- and neuroprotection against ischemia-reperfusion injury. Meclizine increased lactate production and was a potent inducer of the PRC stress program, suggesting that PRC may contribute to the protective effects of meclizine. Finally, c-MYC and PRC were coordinately induced under all conditions tested, implicating c-MYC in the biological response to metabolic stress. The results suggest a general role for PRC in the adaptive response to cellular dysfunction. | 23364789
 |
PGC-1-related coactivator (PRC), a sensor of metabolic stress, orchestrates a redox-sensitive program of inflammatory gene expression.
Gleyzer, N; Scarpulla, RC
The Journal of biological chemistry
286
39715-25
2010
Abstract anzeigen
PGC-1-related coactivator (PRC) is a growth-regulated transcriptional cofactor that activates many nuclear genes specifying mitochondrial respiratory function. Stable PRC silencing in U2OS cells results in a complex phenotype typical of mitochondrial dysfunction including abundant abnormal mitochondria, reduced respiratory subunit expression, diminished respiratory enzymes and ATP levels, and elevated lactate production. The PRC response to metabolic stress was investigated by subjecting cells to metabolic insults including treatment with the uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP), expression of a dominant negative allele of nuclear respiratory factor 1 (NRF-1), and glucose deprivation. These treatments led to constitutively elevated PRC protein levels, a departure from its normal transient expression upon the initiation of cell growth. A microarray screen identified 45 genes that require PRC for their induction by CCCP. A subset of these genes specific to inflammation and cell stress was also induced by dominant negative NRF-1 and by glucose deprivation, suggesting that diverse metabolic insults converge on the same PRC-dependent inflammatory program. The PRC-dependent inflammatory response was inhibited by N-acetylcysteine, suggesting that PRC may contribute to the inflammatory microenvironment linked to oxidant signaling. The induction of this PRC-dependent program may be an early event in adaptations linked to cancer and degenerative diseases. | 21937425
 |
PGC-1-related coactivator complexes with HCF-1 and NRF-2beta in mediating NRF-2(GABP)-dependent respiratory gene expression.
Vercauteren, K; Gleyzer, N; Scarpulla, RC
The Journal of biological chemistry
283
12102-11
2008
Abstract anzeigen
The PGC-1 family of regulated coactivators (PGC-1alpha, PGC-1beta, and PRC) plays an important role in directing respiratory gene expression in response to environmental signals. Here, we show that PRC and PGC-1alpha differ in their interactions with nuclear hormone receptors but are highly similar in their direct binding to several nuclear transcription factors implicated in the expression of the respiratory chain. Surprisingly, neither coactivator binds NRF-2(GABP), a multisubunit transcriptional activator associated with the expression of many respiratory genes. However, the NRF-2 subunits and PRC are co-immunoprecipitated from cell extracts indicating that the two proteins exist in a complex in vivo. Several lines of evidence indicate that HCF-1 (host cell factor 1), a major chromatin component, mediates the association between PRC and NRF-2. Both PRC and NRF-2beta bind HCF-1 in vitro, and the molecular determinants required for the interactions of each with HCF-1 are also required for PRC trans-activation through promoter-bound NRF-2. These determinants include a consensus HCF-1 binding site on PRC and the NRF-2 activation domain. In addition, PRC and NRF-2beta can complex with HCF-1 in vivo, and all three associate with NRF-2-dependent nuclear genes that direct the expression of the mitochondrial transcription factors, TFB1M and TFB2M. Finally, short hairpin RNA-mediated knock down of PRC protein levels leads to reduced expression of TFB2M mRNA and mitochondrial transcripts for cytochrome oxidase II (COXII) and cytochrome b. These changes in gene expression coincide with a marked reduction in cytochrome oxidase activity. The results are consistent with a pathway whereby PRC regulates NRF-2-dependent genes through a multiprotein complex involving HCF-1. | 18343819
 |
Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators.
Gleyzer, N; Vercauteren, K; Scarpulla, RC
Molecular and cellular biology
25
1354-66
2004
Abstract anzeigen
In vertebrates, mitochondrial DNA (mtDNA) transcription is initiated bidirectionally from closely spaced promoters, HSP and LSP, within the D-loop regulatory region. Early studies demonstrated that mtDNA transcription requires mitochondrial RNA polymerase and Tfam, a DNA binding stimulatory factor that is required for mtDNA maintenance. Recently, mitochondrial transcription specificity factors (TFB1M and TFB2M), which markedly enhance mtDNA transcription in the presence of Tfam and mitochondrial RNA polymerase, have been identified in mammalian cells. Here, we establish that the expression of human TFB1M and TFB2M promoters is governed by nuclear respiratory factors (NRF-1 and NRF-2), key transcription factors implicated in mitochondrial biogenesis. In addition, we show that NRF recognition sites within both TFB promoters are required for maximal trans activation by the PGC-1 family coactivators, PGC-1alpha and PRC. The physiological induction of these coactivators has been associated with the integration of NRFs and other transcription factors in a program of mitochondrial biogenesis. Finally, we demonstrate that the TFB genes are up-regulated along with Tfam and either PGC-1alpha or PRC in cellular systems where mitochondrial biogenesis is induced. Moreover, ectopic expression of PGC-1alpha is sufficient to induce the coordinate expression of all three nucleus-encoded mitochondrial transcription factors along with nuclear and mitochondrial respiratory subunits. These results support the conclusion that the coordinate regulation of nucleus-encoded mitochondrial transcription factors by NRFs and PGC-1 family coactivators is essential to the control of mitochondrial biogenesis. | 15684387
 |