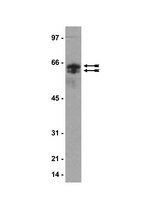
Citrullination of proteins: a common post-translational modification pathway induced by different nanoparticles in vitro and in vivo.
Mohamed, BM; Verma, NK; Davies, AM; McGowan, A; Crosbie-Staunton, K; Prina-Mello, A; Kelleher, D; Botting, CH; Causey, CP; Thompson, PR; Pruijn, GJ; Kisin, ER; Tkach, AV; Shvedova, AA; Volkov, Y
Nanomedicine (London, England)
7
1181-95
2011
Abstract anzeigen
Rapidly expanding manufacture and use of nanomaterials emphasize the requirements for thorough assessment of health outcomes associated with novel applications. Post-translational protein modifications catalyzed by Ca(2+)-dependent peptidylargininedeiminases have been shown to trigger immune responses including autoantibody generation, a hallmark of immune complexes deposition in rheumatoid arthritis. Therefore, the aim of the study was to assess if nanoparticles are able to promote protein citrullination.Human A549 and THP-1 cells were exposed to silicon dioxide, carbon black or single-walled carbon nanotubes. C57BL/6 mice were exposed to respirable single-walled carbon nanotubes. Protein citrullination, peptidylargininedeiminases activity and target proteins were evaluated.The studied nanoparticles induced protein citrullination both in cultured human cells and mouse lung tissues. Citrullination occurred via the peptidylargininedeiminase-dependent mechanism. Cytokeratines 7, 8, 18 and plectins were identified as intracellular citrullination targets.Nanoparticle exposure facilitated post-translational citrullination of proteins. | Western Blotting | 22625207
 |
Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization.
Kopp, HG; Hooper, AT; Broekman, MJ; Avecilla, ST; Petit, I; Luo, M; Milde, T; Ramos, CA; Zhang, F; Kopp, T; Bornstein, P; Jin, DK; Marcus, AJ; Rafii, S
The Journal of clinical investigation
116
3277-91
2005
Abstract anzeigen
Thrombopoietic cells may differentially promote or inhibit tissue vascularization by releasing both pro- and antiangiogenic factors. However, the molecular determinants controlling the angiogenic phenotype of thrombopoietic cells remain unknown. Here, we show that expression and release of thrombospondins (TSPs) by megakaryocytes and platelets function as a major antiangiogenic switch. TSPs inhibited thrombopoiesis, diminished bone marrow microvascular reconstruction following myelosuppression, and limited the extent of revascularization in a model of hind limb ischemia. We demonstrate that thrombopoietic recovery following myelosuppression was significantly enhanced in mice deficient in both TSP1 and TSP2 (TSP-DKO mice) in comparison with WT mice. Megakaryocyte and platelet levels in TSP-DKO mice were rapidly restored, thereby accelerating revascularization of myelosuppressed bone marrow and ischemic hind limbs. In addition, thrombopoietic cells derived from TSP-DKO mice were more effective in supporting neoangiogenesis in Matrigel plugs. The proangiogenic activity of TSP-DKO thrombopoietic cells was mediated through activation of MMP-9 and enhanced release of stromal cell-derived factor 1. Thus, TSP-deficient thrombopoietic cells function as proangiogenic agents, accelerating hemangiogenesis within the marrow and revascularization of ischemic hind limbs. As such, interference with the release of cellular stores of TSPs may be clinically effective in augmenting neoangiogenesis. Volltextartikel | | 17143334
 |
Sequential reorganization of cornified cell keratin filaments involving filaggrin-mediated compaction and keratin 1 deimination.
Ishida-Yamamoto, Akemi, et al.
J. Invest. Dermatol., 118: 282-7 (2002)
2002
Abstract anzeigen
The final step of keratinocyte differentiation, transition from the granular cells to the cornified cells, involves various post-translational modifications that include deimination of arginine residues. Major deiminated epidermal proteins are derived from K1. Two preferred deimination sites were identified in mouse K1, one in the V1 and the other in the V2 subdomains. An antibody against the deiminated peptide sequence in the V2 subdomain recognized not only deiminated mouse K1 but also deiminated human K1. In this study we analyzed distribution of deiminated K1 in normal human skin and in bullous congenital ichthyosiform erythroderma at light and electron microscopic levels. In normal skin the first few (1-3) cornified cell layers were positive for filaggrin and negative for the antibody against deiminated mouse K1 peptide, whereas the more superficial cells were negative for filaggrin and strongly positive for the antibody against deiminated mouse K1 peptide, indicating slightly delayed onset of K1 deimination at the initial stage of cornification. The clumped keratin in bullous congenital ichthyosiform erythroderma that was not properly compacted with filaggrin was poorly positive to the antibody against deiminated mouse K1 peptide. In addition, K1 derivatives in bullous congenital ichthyosiform erythroderma reacted poorly with the antibody against deiminated mouse K1 peptide compared with the normal control in immunoblot analyses. Our results suggest sequential reorganization of cornified cell keratin filaments involving filaggrin-mediated compaction and K1 deimination. Abnormal keratin aggregation in bullous congenital ichthyosiform erythroderma is likely to disturb the normal deimination of K1. | | 11841545
 |
Decreased deiminated keratin K1 in psoriatic hyperproliferative epidermis.
Ishida-Yamamoto, A, et al.
J. Invest. Dermatol., 114: 701-5 (2000)
1999
Abstract anzeigen
Citrulline-containing proteins, mainly originating from keratin K1 and formed by enzymatic deimination of arginine residues, have been identified in the cornified layers of human epidermis. We analyzed the localization and nature of the deiminated proteins in psoriatic epidermis. Immunostaining based on chemical modification of citrulline residues showed that the normal and psoriatic uninvolved epidermis contained deiminated proteins diffusely in the cornified cell layer, whereas the involved epidermis had no detectable or markedly reduced levels of deiminated proteins. Immunolabeling with polyclonal antibodies against a synthetic citrulline-containing peptide corresponding to a deiminated sequence of mouse K1 also suggested markedly decreased deiminated K1 in psoriatic involved lesions. Keratin analyses indicated that deiminated K1 present in normal and psoriatic uninvolved epidermis was not detected in the psoriatic involved epidermis. Double staining with a monoclonal antibody, 34betaB4, and the polyclonal antibodies demonstrated that epidermis with low suprabasal keratin expression was negative for deiminated K1. In contrast, intralesional acrosyringia showing decreased suprabasal keratin immunoreactivity like that of the surrounding psoriatic epidermis showed strong deiminated K1 staining. This suggests that abnormal keratin deimination is restricted to the psoriatic hyperproliferative epidermis, without affecting sweat ductal epithelia. | | 10733676
 |
Studies on specificity of peptidylarginine deiminase reactions using an immunochemical probe that recognizes an enzymatically deiminated partial sequence of mouse keratin K1.
Senshu, T, et al.
J. Dermatol. Sci., 21: 113-26 (1999)
1998
Abstract anzeigen
Citrulline residues are detected in keratins and filaggrin in the cornified layers of mammalian epidermis. Such citrulline residues are formed by the enzymatic deimination of arginine residues by peptidylarginine deiminases (EC 3.5.3.15). Major deiminated keratins are derived from keratin K1. Two arginine residues identified as preferred deimination sites in mouse K1 are located in its V subdomains. To develop an immunochemical probe which recognizes the deiminated peptide sequence specifically, we enzymatically deiminated an undecapeptide corresponding to the deiminated peptide sequence identified in the V2 subdomain for immunizing rabbits. An IgG fraction obtained from the antiserum was affinity-purified using an immobilized peptide column. The affinity-purified IgG showed high specificity towards partially degraded keratin K1 obtained from the cornified layer of 3-day-old mouse epidermis. It also yielded intense signals of unidentified minor components localized in the cornified layers of late embryonic and early postnatal mouse epidermis. Comparative studies using different types of the enzymes suggested that peptidylarginine deiminase type I acted on the arginine residue in the V2 subdomain of keratin K1 more readily than peptidylarginine deiminase type II. The data are discussed in conjunction with possible factors influencing the specificity of the enzyme reaction. | | 10511480
 |
Preferential deimination of keratin K1 and filaggrin during the terminal differentiation of human epidermis.
Senshu, T, et al.
Biochem. Biophys. Res. Commun., 225: 712-9 (1996)
1996
Abstract anzeigen
The upper layers of mammalian epidermis contain citrulline-containing proteins formed by enzymatic deimination of arginine residues. To study the role of protein deimination in epidermal differentiation, we identified deiminated proteins extracted from human epidermis. Major deiminated proteins were identified as partially degraded keratin K1, while those from keratin K10 and a highly heterogeneous mixture of deiminated filaggrin isomers were detected as minor components. Deiminated keratins were recovered in a fraction enriched with keratins from the cornified layers. The subsequent immunohistochemical study showed that deiminated proteins were localized mainly in the lowermost cornified layer, but not in the granular layer. These data suggested that partially degraded/disulfide-cross-linked keratin K1 was preferentially deiminated during the terminal stages of epidermal differentiation. We therefore speculated that the protein deimination might influence the interaction of basic K1 with its acidic partner K10, pre-existent K5/K14 networks or keratin-associated protein filaggrin. | | 8780679
 |