516535 Sigma-AldrichPERK Inhibitor I, GSK2606414 - CAS 1337531-89-1 - Calbiochem
PERK Inhibitor I, GSK2606414, CAS 1337531-89-1, is a cell-permeable, highly potent EIF2AK3/PERK inhibitor (IC50 = 0.4 nM) that targets PERK in its inactive DFG conformation at the ATP-binding region.
More>> PERK Inhibitor I, GSK2606414, CAS 1337531-89-1, is a cell-permeable, highly potent EIF2AK3/PERK inhibitor (IC50 = 0.4 nM) that targets PERK in its inactive DFG conformation at the ATP-binding region. Less<<Synonyms: DDR2 Inhibitor I, EIF2AK1 Inhibitor I, EIF2AK2 Inhibitor II, HRI Inhibitor I, MLCK Inhibitor VI, MLK2/MAP3K10 Inhibitor I, PKR-like ER Kinase Inhibitor I, Aurora Kinase Inhibitor IX, BRK Inhibitor I, c-Kit Inhibitor III, Mer RTK Inhibitor II, EIF2AK3 Inhibitor I, PKR Inhibitor II, MERTK Inhibitor II, RP38 Inhibitor II, TAM Family RTK Inhibitor II, Double-stranded RNA-activated Protein Kinase Inhibitor II, Double-stranded RNA-dependent Protein Kinase Inhibitor II, 7-Methyl-5-(1-((3-trifluoromethyl)phenyl)acetyl)-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine, 1-(5-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)indolin-1-yl)-2-(3-(trifluoromethyl)phenyl)ethanone
Recommended Products
Přehled
| Replacement Information |
|---|
Tabulka spec. kláve
| CAS # | Empirical Formula |
|---|---|
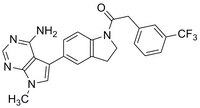
| 1337531-89-1 | C₂₄H₂₀F₃N₅O |
Products
| Katalogové číslo | Balení | ks/bal. | |
|---|---|---|---|
| 516535-5MG | Skleněná láhev | 5 mg |
| Description | |
|---|---|
| Overview | A cell-permeable pyrrolopyrimidamine compound that acts as a highly potent EIF2AK3/PERK inhibitor (IC50 = 0.4 nM; [ATP] = 5 µM) by targeting PERK in its inactive DFG conformation at the ATP-binding region, while displaying ≥385-fold selectivity over c-Kit, Aurora B, BRK, HRI/EIF2AK1, MLK2/MAP3K10, c-MER, DDR2, PKR/EIF2AK2, and MLCK2/MYLK2 (IC50 = 154, 407, 412, 420, 452, 474, 524, 696, and 701 nM, respectively) and little activity against more than 280 other kinases (IC50 >1 µM). Shown to block ER stress-induced PERK autophosphorylation following Thapsigargin (Cat. No. 586005) addition in A549 cultures in vitro (by 100% at ≤30 nM; 60 min preincubation) and effectively retard PxBC-3 tumor growth in mice in vivo (50 and 150 mg/kg/12 h p.o.). Also available as a 25 mM solution in DMSO (Cat. No. 508340). |
| Catalogue Number | 516535 |
| Brand Family | Calbiochem® |
| Synonyms | DDR2 Inhibitor I, EIF2AK1 Inhibitor I, EIF2AK2 Inhibitor II, HRI Inhibitor I, MLCK Inhibitor VI, MLK2/MAP3K10 Inhibitor I, PKR-like ER Kinase Inhibitor I, Aurora Kinase Inhibitor IX, BRK Inhibitor I, c-Kit Inhibitor III, Mer RTK Inhibitor II, EIF2AK3 Inhibitor I, PKR Inhibitor II, MERTK Inhibitor II, RP38 Inhibitor II, TAM Family RTK Inhibitor II, Double-stranded RNA-activated Protein Kinase Inhibitor II, Double-stranded RNA-dependent Protein Kinase Inhibitor II, 7-Methyl-5-(1-((3-trifluoromethyl)phenyl)acetyl)-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine, 1-(5-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)indolin-1-yl)-2-(3-(trifluoromethyl)phenyl)ethanone |
| References | |
|---|---|
| References | Moreno, J.A., et al. 2013. Sci Transl. Med.<\i> 5, 1. Axten, J.M., et al. 2012. J. Med. Chem. 55, 7193. |
| Product Information | |
|---|---|
| CAS number | 1337531-89-1 |
| Form | White powder |
| Hill Formula | C₂₄H₂₀F₃N₅O |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ200 |
| Biological Information | |
|---|---|
| Primary Target | Perk |
| Primary Target IC<sub>50</sub> | 0.4 nM against EIF2AK3/PERK-catalyzed EIF2&alpha |
| Purity | ≥99% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalogové číslo | GTIN |
| 516535-5MG | 04055977198126 |
Documentation
PERK Inhibitor I, GSK2606414 - CAS 1337531-89-1 - Calbiochem MSDS
| Title |
|---|
PERK Inhibitor I, GSK2606414 - CAS 1337531-89-1 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 516535 |
References
| Přehled odkazů |
|---|
| Moreno, J.A., et al. 2013. Sci Transl. Med.<\i> 5, 1. Axten, J.M., et al. 2012. J. Med. Chem. 55, 7193. |
| Data Sheet | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|