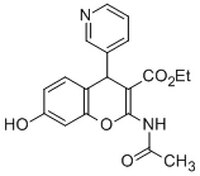
530613 Sigma-AldrichIRAP Inhibitor, HFI-419 - CAS 1110650-72-0 - Calbiochem
A substrate competitive inhibitor of insulin-regulated aminopeptidase (IRAP) activity (Ki = 0.48 µM against 25 µM Leu-AMC).
More>> A substrate competitive inhibitor of insulin-regulated aminopeptidase (IRAP) activity (Ki = 0.48 µM against 25 µM Leu-AMC). Less<<Synonyms: (±)-Ethyl-2-acetamido-7-hydroxy-4-(pyridin-3-yl)-4H-chromene-3-carboxylate, HFI419, Insulin-Regulated Aminopeptidase Inhibitor
Recommended Products
Přehled
| Replacement Information |
|---|
Tabulka spec. kláve
| CAS # | Empirical Formula |
|---|---|
| 1110650-72-0 | C₁₉H₁₈N₂O₅ |
Products
| Katalogové číslo | Balení | ks/bal. | |
|---|---|---|---|
| 5.30613.0001 | Skleněná láhev | 10 mg |
| Product Information | |
|---|---|
| CAS number | 1110650-72-0 |
| Form | Off-white powder |
| Hill Formula | C₁₉H₁₈N₂O₅ |
| Chemical formula | C₁₉H₁₈N₂O₅ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | IRAP |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalogové číslo | GTIN |
| 5.30613.0001 | 04055977260946 |
Documentation
IRAP Inhibitor, HFI-419 - CAS 1110650-72-0 - Calbiochem MSDS
| Title |
|---|
References
| Přehled odkazů |
|---|
| Mountford, S.J., et al. 2014. J. Med. Chem. 57, 1368. Albiston, A.L., et al. 2011. Br. J. Pharmacol. 164, 37. Albiston, A.L., et al. 2010. Mol. Pharmacol. 78, 600. Albiston, A.L., et al. 2008. FASEB J. 22, 4209. |