Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes.
Blind, RD; Garabedian, MJ
The Journal of steroid biochemistry and molecular biology
109
150-7
2008
Zobrazit abstrakt
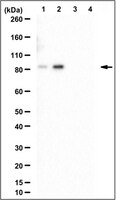
The human glucocorticoid receptor (GR) is phosphorylated on its N-terminus at three major sites (S203, S211 and S226) within activation function 1 (AF1). Although GR has been shown to assemble at glucocorticoid responsive elements (GREs) in the presence of hormone, the timing and specificity of GR phospho-isoform recruitment to receptor target genes has not been established. Using chromatin immunoprecipitation (ChIP) and GR phosphorylation site-specific antibodies, we examined GR phospho-isoform recruitment to several glucocorticoid-induced genes including tyrosine aminotransferase (tat) and sulfonyltransferase-1A1 (sult) in rat hepatoma cells, and the glucocorticoid-induced leucine zipper (gilz) gene in human U2OS cells. GR P-S211 and GR P-S226 isoforms were efficiently recruited to the tat, sult and gilz GREs in a hormone-dependent manner. In contrast, the GR P-S203 isoform displayed no significant recruitment to any GREs of the genes analyzed, consistent with its lack of nuclear accumulation. Interestingly, the kinetics of GR P-S211 and GR P-S226 recruitment differed among genes. Our findings indicate that GR phospho-isoforms selectively occupy GR target genes, and suggests gene specific requirements for GR phosphorylation in receptor-dependent transcriptional activation. | 18304804
 |
Glucocorticoid receptor phosphorylation differentially affects target gene expression.
Chen, W; Dang, T; Blind, RD; Wang, Z; Cavasotto, CN; Hittelman, AB; Rogatsky, I; Logan, SK; Garabedian, MJ
Molecular endocrinology (Baltimore, Md.)
22
1754-66
2008
Zobrazit abstrakt
The glucocorticoid receptor (GR) is phosphorylated at multiple sites within its N terminus (S203, S211, S226), yet the role of phosphorylation in receptor function is not understood. Using a range of agonists and GR phosphorylation site-specific antibodies, we demonstrated that GR transcriptional activation is greatest when the relative phosphorylation of S211 exceeds that of S226. Consistent with this finding, a replacement of S226 with an alanine enhances GR transcriptional response. Using a battery of compounds that perturb different signaling pathways, we found that BAPTA-AM, a chelator of intracellular divalent cations, and curcumin, a natural product with antiinflammatory properties, reduced hormone-dependent phosphorylation at S211. This change in GR phosphorylation was associated with its decreased nuclear retention and transcriptional activation. Molecular modeling suggests that GR S211 phosphorylation promotes a conformational change, which exposes a novel surface potentially facilitating cofactor interaction. Indeed, S211 phosphorylation enhances GR interaction with MED14 (vitamin D receptor interacting protein 150). Interestingly, in U2OS cells expressing a nonphosphorylated GR mutant S211A, the expression of IGF-binding protein 1 and interferon regulatory factor 8, both MED14-dependent GR target genes, was reduced relative to cells expressing wild-type receptor across a broad range of hormone concentrations. In contrast, the induction of glucocorticoid-induced leucine zipper, a MED14-independent GR target, was similar in S211A- and wild-type GR-expressing cells at high hormone levels, but was reduced in S211A cells at low hormone concentrations, suggesting a link between GR phosphorylation, MED14 involvement, and receptor occupancy. Phosphorylation also affected the magnitude of repression by GR in a gene-selective manner. Thus, GR phosphorylation at S211 and S226 determines GR transcriptional response by modifying cofactor interaction. Furthermore, the effect of GR S211 phosphorylation is gene specific and, in some cases, dependent upon the amount of activated receptor. | 18483179
 |