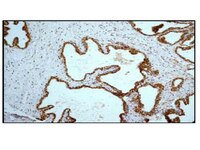
Characterization of Aldh2 (-/-) mice as an age-related model of cognitive impairment and Alzheimer's disease.
D'Souza, Y; Elharram, A; Soon-Shiong, R; Andrew, RD; Bennett, BM
Molecular brain
8
27
2015
Zobrazit abstrakt
The study of late-onset/age-related Alzheimer's disease (AD)(sporadic AD, 95% of AD cases) has been hampered by a paucity of animal models. Oxidative stress is considered a causative factor in late onset/age-related AD, and aldehyde dehydrogenase 2 (ALDH2) is important for the catabolism of toxic aldehydes associated with oxidative stress. One such toxic aldehyde, the lipid peroxidation product 4-hydroxynonenal (HNE), accumulates in AD brain and is associated with AD pathology. Given this linkage, we hypothesized that in mice lacking ALDH2, there would be increases in HNE and the appearance of AD-like pathological changes.Changes in relevant AD markers in Aldh2 (-/-) mice and their wildtype littermates were assessed over a 1 year period. Marked increases in HNE adducts arise in hippocampi from Aldh2 (-/-) mice, as well as age-related increases in amyloid-beta, p-tau, and activated caspases. Also observed were age-related decreases in pGSK3β, PSD95, synaptophysin, CREB and pCREB. Age-related memory deficits in the novel object recognition and Y maze tasks begin at 3.5-4 months and are maximal at 6.5-7 months. There was decreased performance in the Morris Water Maze task in 6 month old Aldh2 (-/-) mice. These mice exhibited endothelial dysfunction, increased amyloid-beta in cerebral microvessels, decreases in carbachol-induced pCREB and pERK formation in hippocampal slices, and brain atrophy. These AD-associated pathological changes are rarely observed as a constellation in current AD animal models.We believe that this new model of age-related cognitive impairment will provide new insight into the pathogenesis and molecular/cellular mechanisms driving neurodegenerative diseases of aging such as AD, and will prove useful for assessing the efficacy of therapeutic agents for improving memory and for slowing, preventing, or reversing AD progression. | | | 25910195
 |
Region and context-specific intracellular responses associated with cocaine-induced conditioned place preference expression.
Nygard, SK; Klambatsen, A; Balouch, B; Quinones-Jenab, V; Jenab, S
Neuroscience
287
1-8
2015
Zobrazit abstrakt
The development and maintenance of cocaine addiction depend heavily on learned reward-environment associations that can induce drug-seeking behavior and relapse. Understanding the mechanisms underlying these cue-induced conditioned responses is important for relapse prevention. To test whether intracellular responses measured after cocaine conditioned place preference (CPP) expression are context-dependent, we re-exposed cocaine-treated rats (drug-free) to an environment previously paired with cocaine or saline, 24h after the CPP test. After 8 days of cocaine CPP training with one of two cocaine doses (5mg/kg or 20mg/kg, i.p.), CPP was expressed only after conditioning with the higher cocaine dose. In CPP expressing rats, locomotor responses after re-exposure to the cocaine-chamber were greater than in rats re-exposed to the saline-paired chamber. Nucleus Accumbens (NAc) phosphorylated ERK (pERK) levels were increased after re-exposure to the cocaine-paired, but not the saline-paired chamber, regardless of whether or not CPP behavior was expressed. Caudate Putamen (CPu) pERK and FosB protein levels increased after re-exposure to the cocaine chamber only after conditioning with the higher cocaine dose. Conversely, the higher cocaine dose, independent of environment, resulted in increased NAc FosB, ΔFosB and phosphorylated CREB (pCREB) protein levels compared to those conditioned with 5mg/kg cocaine (non-CPP-expressing). Our results suggest that NAc ERK phosphorylation may be involved with retrieving the contextual information of a cocaine-association, without necessarily motivating the expression of CPP behavior. Additionally, we show distinct patterns of intracellular responses in the NAc and CPu indicating a region-specific role for pERK/pCREB/FosB intracellular signaling in the retrieval of cocaine-context associations. | | | 25522720
 |
Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area.
Koo, JW; Mazei-Robison, MS; LaPlant, Q; Egervari, G; Braunscheidel, KM; Adank, DN; Ferguson, D; Feng, J; Sun, H; Scobie, KN; Damez-Werno, DM; Ribeiro, E; Peña, CJ; Walker, D; Bagot, RC; Cahill, ME; Anderson, SA; Labonté, B; Hodes, GE; Browne, H; Chadwick, B; Robison, AJ; Vialou, VF; Dias, C; Lorsch, Z; Mouzon, E; Lobo, MK; Dietz, DM; Russo, SJ; Neve, RL; Hurd, YL; Nestler, EJ
Nature neuroscience
18
415-22
2015
Zobrazit abstrakt
Brain-derived neurotrophic factor (BDNF) has a crucial role in modulating neural and behavioral plasticity to drugs of abuse. We found a persistent downregulation of exon-specific Bdnf expression in the ventral tegmental area (VTA) in response to chronic opiate exposure, which was mediated by specific epigenetic modifications at the corresponding Bdnf gene promoters. Exposure to chronic morphine increased stalling of RNA polymerase II at these Bdnf promoters in VTA and altered permissive and repressive histone modifications and occupancy of their regulatory proteins at the specific promoters. Furthermore, we found that morphine suppressed binding of phospho-CREB (cAMP response element binding protein) to Bdnf promoters in VTA, which resulted from enrichment of trimethylated H3K27 at the promoters, and that decreased NURR1 (nuclear receptor related-1) expression also contributed to Bdnf repression and associated behavioral plasticity to morphine. Our findings suggest previously unknown epigenetic mechanisms of morphine-induced molecular and behavioral neuroadaptations. | | | 25643298
 |
Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice.
Villeda, SA; Plambeck, KE; Middeldorp, J; Castellano, JM; Mosher, KI; Luo, J; Smith, LK; Bieri, G; Lin, K; Berdnik, D; Wabl, R; Udeochu, J; Wheatley, EG; Zou, B; Simmons, DA; Xie, XS; Longo, FM; Wyss-Coray, T
Nature medicine
20
659-63
2014
Zobrazit abstrakt
As human lifespan increases, a greater fraction of the population is suffering from age-related cognitive impairments, making it important to elucidate a means to combat the effects of aging. Here we report that exposure of an aged animal to young blood can counteract and reverse pre-existing effects of brain aging at the molecular, structural, functional and cognitive level. Genome-wide microarray analysis of heterochronic parabionts--in which circulatory systems of young and aged animals are connected--identified synaptic plasticity-related transcriptional changes in the hippocampus of aged mice. Dendritic spine density of mature neurons increased and synaptic plasticity improved in the hippocampus of aged heterochronic parabionts. At the cognitive level, systemic administration of young blood plasma into aged mice improved age-related cognitive impairments in both contextual fear conditioning and spatial learning and memory. Structural and cognitive enhancements elicited by exposure to young blood are mediated, in part, by activation of the cyclic AMP response element binding protein (Creb) in the aged hippocampus. Our data indicate that exposure of aged mice to young blood late in life is capable of rejuvenating synaptic plasticity and improving cognitive function. | Immunohistochemistry | Mouse | 24793238
 |
Inhibition of phosphodiesterase10A attenuates morphine-induced conditioned place preference.
Mu, Y; Ren, Z; Jia, J; Gao, B; Zheng, L; Wang, G; Friedman, E; Zhen, X
Molecular brain
7
70
2014
Zobrazit abstrakt
Phosphodiesterase (PDE) 10A is selectively expressed in medium spiny neurons of the striatum. Nucleus accumbens (NAc) is a key region that mediates drug reward and addiction-related behaviors. To investigate the potential role of PDE10A in the reinforcement properties of morphine, we tested the effect of MP-10, a selective inhibitor of PDE10A, on acquisition, expression, and extinction of morphine-induced conditioned place preference (CPP).The results show that 2.5 mg/kg MP-10, administered subcutaneously, significantly inhibited the acquisition of morphine-induced CPP. The same dose of MP-10 alone did not result in the CPP. Moreover, MP-10 did not alter the expression of morphine-induced CPP, but did accelerate the extinction of morphine-induced CPP. Additionally, chronic treatment with 2.5 mg/kg MP-10 decreased expression of phosphorylated CREB (pCREB), activated cAMP response element binding protein, in dorsomedial striatum, in shell of NAc, and in anterior cingulate cortex (ACC) as well as decreased expression of ΔFosB in the shell of NAc and ACC.The results suggest that inhibition of PDE10A may have therapeutic potential in the treatment of opioid addiction. | Immunofluorescence | | 25252626
 |
AAD-2004 Attenuates Progressive Neuronal Loss in the Brain of Tg-betaCTF99/B6 Mouse Model of Alzheimer Disease.
Baek, IS; Kim, TK; Seo, JS; Lee, KW; Lee, YA; Cho, J; Gwag, BJ; Han, PL
Experimental neurobiology
22
31-7
2013
Zobrazit abstrakt
Alzheimer's disease (AD) is a neurodegenerative disease that proceeds with the age-dependent neuronal loss, an irreversible event which causes severe cognitive and psychiatric devastations. In the present study, we investigated whether the compound, AAD-2004 [2-hydroxy-5-[2-(4-trifluoromethylphenyl)-ethylaminobenzoic acid] which has anti-oxidant and anti-inflammatory properties, is beneficial for the brain of Tg-betaCTF99/B6 mice, a murine AD model that was recently developed to display age-dependent neuronal loss and neuritic atrophy in the brain. Administration of AAD-2004 in Tg-betaCTF99/B6 mice from 10 months to 18 months of age completely repressed the accumulation of lipid peroxidation in the brain. AAD-2004 markedly suppressed neuronal loss and neuritic atrophy, and partially reversed depleted expression of calbindin in the brain of Tg-beta-CTF99/B6. These results suggest that AAD-2004 affords neurodegeneration in the brain of AD mouse model. | | | 23585720
 |
A tetra(ethylene glycol) derivative of benzothiazole aniline enhances Ras-mediated spinogenesis.
Megill, A; Lee, T; DiBattista, AM; Song, JM; Spitzer, MH; Rubinshtein, M; Habib, LK; Capule, CC; Mayer, M; Turner, RS; Kirkwood, A; Yang, J; Pak, DT; Lee, HK; Hoe, HS
The Journal of neuroscience : the official journal of the Society for Neuroscience
33
9306-18
2013
Zobrazit abstrakt
The tetra(ethylene glycol) derivative of benzothiazole aniline, BTA-EG4, is a novel amyloid-binding small molecule that can penetrate the blood-brain barrier and protect cells from Aβ-induced toxicity. However, the effects of Aβ-targeting molecules on other cellular processes, including those that modulate synaptic plasticity, remain unknown. We report here that BTA-EG4 decreases Aβ levels, alters cell surface expression of amyloid precursor protein (APP), and improves memory in wild-type mice. Interestingly, the BTA-EG4-mediated behavioral improvement is not correlated with LTP, but with increased spinogenesis. The higher dendritic spine density reflects an increase in the number of functional synapses as determined by increased miniature EPSC (mEPSC) frequency without changes in presynaptic parameters or postsynaptic mEPSC amplitude. Additionally, BTA-EG4 requires APP to regulate dendritic spine density through a Ras signaling-dependent mechanism. Thus, BTA-EG4 may provide broad therapeutic benefits for improving neuronal and cognitive function, and may have implications in neurodegenerative disease therapy. | | | 23719799
 |
Sexually dimorphic intracellular responses after cocaine-induced conditioned place preference expression.
Nygard, SK; Klambatsen, A; Hazim, R; Eltareb, MH; Blank, JC; Chang, AJ; Quinones-Jenab, V; Jenab, S
Brain research
1520
121-33
2013
Zobrazit abstrakt
Sex differences in cocaine's mechanisms of action and behavioral effects have been widely reported. However, little is known about how sex influences intracellular signaling cascades involved with drug-environment associations. We investigated whether ERK/CREB intracellular responses in the mesocorticolimbic circuitry underlying cocaine environmental associations are sexually dimorphic. We used a standard 4 day conditioned place preference (CPP) paradigm using 20mg/kg cocaine-a dose that induced CPP in male and female Fischer rats. In the nucleus accumbens (NAc) following CPP expression, cocaine treated animals showed increased phosphorylated ERK (pERK), phosphorylated CREB (pCREB) and ΔFosB protein levels. In the hippocampus (HIP) and caudate putamen (CPu), pERK and FosB/ΔFosB levels were also increased, respectively. Cocaine females had a larger change in HIP pERK and CPu ΔFosB levels than cocaine males; partly due to lower protein levels in saline female rats when compared to saline males. Prefrontal cortex (PfC) pCREB levels increased in cocaine males, but not females, whereas PfC pERK levels were increased in cocaine females, but not males. CPP scores were positively correlated to NAc pERK, HIP pERK and CPu FosB protein levels, suggesting that similar to males, the ERK/CREB intracellular pathway in mesocorticolimbic regions undergoes cocaine induced neuroplasticity in female rats. However, there seem to be intrinsic (basal) sexual dimorphisms in this pathway that may contribute to responses expressed after cocaine-CPP. Taken together, our results suggest that cellular responses associated with the expression of learned drug-environment associations may play an important role in sex differences in cocaine addiction and relapse. | | | 23665060
 |
M1 muscarinic receptor activation mediates cell death in M1-HEK293 cells.
Graham, ES; Woo, KK; Aalderink, M; Fry, S; Greenwood, JM; Glass, M; Dragunow, M
PloS one
8
e72011
2013
Zobrazit abstrakt
HEK293 cells have been used extensively to generate stable cell lines to study G protein-coupled receptors, such as muscarinic acetylcholine receptors (mAChRs). The activation of M1 mAChRs in various cell types in vitro has been shown to be protective. To further investigate M1 mAChR-mediated cell survival, we generated stable HEK293 cell-lines expressing the human M1 mAChR. M1 mAChRs were efficiently expressed at the cell surface and efficiently internalised within 1 h by carbachol. Carbachol also induced early signalling cascades similar to previous reports. Thus, ectopically expressed M1 receptors behaved in a similar fashion to the native receptor over short time periods of analysis. However, substantial cell death was observed in HEK293-M1 cells within 24 h after carbachol application. Death was only observed in HEK cells expressing M1 receptors and fully blocked by M1 antagonists. M1 mAChR-stimulation mediated prolonged activation of the MEK-ERK pathway and resulted in prolonged induction of the transcription factor EGR-1 (greater than 24 h). Blockade of ERK signalling with U0126 did not reduce M1 mAChR-mediated cell-death significantly but inhibited the acute induction of EGR-1. We investigated the time-course of cell death using time-lapse microscopy and xCELLigence technology. Both revealed the M1 mAChR cytotoxicity occurs within several hours of M1 activation. The xCELLigence assay also confirmed that the ERK pathway was not involved in cell-death. Interestingly, the MEK blocker did reduce carbachol-mediated cleaved caspase 3 expression in HEK293-M1 cells. The HEK293 cell line is a widely used pharmacological tool for studying G-protein coupled receptors, including mAChRs. Our results highlight the importance of investigating the longer term fate of these cells in short term signalling studies. Identifying how and why activation of the M1 mAChR signals apoptosis in these cells may lead to a better understanding of how mAChRs regulate cell-fate decisions. | | | 24023725
 |
Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification.
Regard, JB; Malhotra, D; Gvozdenovic-Jeremic, J; Josey, M; Chen, M; Weinstein, LS; Lu, J; Shore, EM; Kaplan, FS; Yang, Y
Nature medicine
19
1505-12
2013
Zobrazit abstrakt
Heterotopic ossification, the pathologic formation of extraskeletal bone, occurs as a common complication of trauma or in genetic disorders and can be disabling and lethal. However, the underlying molecular mechanisms are largely unknown. Here we demonstrate that Gαs restricts bone formation to the skeleton by inhibiting Hedgehog signaling in mesenchymal progenitor cells. In progressive osseous heteroplasia, a human disease caused by null mutations in GNAS, which encodes Gαs, Hedgehog signaling is upregulated in ectopic osteoblasts and progenitor cells. In animal models, we show that genetically-mediated ectopic Hedgehog signaling is sufficient to induce heterotopic ossification, whereas inhibition of this signaling pathway by genetic or pharmacological means strongly reduces the severity of this condition. As our previous work has shown that GNAS gain-of-function mutations upregulate WNT-β-catenin signaling in osteoblast progenitor cells, resulting in their defective differentiation and fibrous dysplasia, we identify Gαs as a key regulator of proper osteoblast differentiation through its maintenance of a balance between the Wnt-β-catenin and Hedgehog pathways. Also, given the results here of the pharmacological studies in our mouse model, we propose that Hedgehog inhibitors currently used in the clinic for other conditions, such as cancer, may possibly be repurposed for treating heterotopic ossification and other diseases caused by GNAS inactivation. | Western Blotting | | 24076664
 |