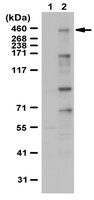
Mitosis inhibits DNA double-strand break repair to guard against telomere fusions.
Orthwein, A; Fradet-Turcotte, A; Noordermeer, SM; Canny, MD; Brun, CM; Strecker, J; Escribano-Diaz, C; Durocher, D
Science (New York, N.Y.)
344
189-93
2014
Zobrazit abstrakt
Mitotic cells inactivate DNA double-strand break (DSB) repair, but the rationale behind this suppression remains unknown. Here, we unravel how mitosis blocks DSB repair and determine the consequences of repair reactivation. Mitotic kinases phosphorylate the E3 ubiquitin ligase RNF8 and the nonhomologous end joining factor 53BP1 to inhibit their recruitment to DSB-flanking chromatin. Restoration of RNF8 and 53BP1 accumulation at mitotic DSB sites activates DNA repair but is, paradoxically, deleterious. Aberrantly controlled mitotic DSB repair leads to Aurora B kinase-dependent sister telomere fusions that produce dicentric chromosomes and aneuploidy, especially in the presence of exogenous genotoxic stress. We conclude that the capacity of mitotic DSB repair to destabilize the genome explains the necessity for its suppression during mitosis, principally due to the fusogenic potential of mitotic telomeres. | 24652939
 |
Dephosphorylation enables the recruitment of 53BP1 to double-strand DNA breaks.
Lee, DH; Acharya, SS; Kwon, M; Drane, P; Guan, Y; Adelmant, G; Kalev, P; Shah, J; Pellman, D; Marto, JA; Chowdhury, D
Molecular cell
54
512-25
2014
Zobrazit abstrakt
Excluding 53BP1 from chromatin is required to attenuate the DNA damage response during mitosis, yet the functional relevance and regulation of this exclusion are unclear. Here we show that 53BP1 is phosphorylated during mitosis on two residues, T1609 and S1618, located in its well-conserved ubiquitination-dependent recruitment (UDR) motif. Phosphorylating these sites blocks the interaction of the UDR motif with mononuclesomes containing ubiquitinated histone H2A and impedes binding of 53BP1 to mitotic chromatin. Ectopic recruitment of 53BP1-T1609A/S1618A to mitotic DNA lesions was associated with significant mitotic defects that could be reversed by inhibiting nonhomologous end-joining. We also reveal that protein phosphatase complex PP4C/R3β dephosphorylates T1609 and S1618 to allow the recruitment of 53BP1 to chromatin in G1 phase. Our results identify key sites of 53BP1 phosphorylation during mitosis, identify the counteracting phosphatase complex that restores the potential for DDR during interphase, and establish the physiological importance of this regulation. | 24703952
 |