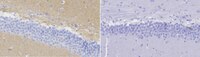
Inner retinal change in a novel rd1-FTL mouse model of retinal degeneration.
Greferath, U; Anderson, EE; Jobling, AI; Vessey, KA; Martinez, G; de Iongh, RU; Kalloniatis, M; Fletcher, EL
Frontiers in cellular neuroscience
9
293
2015
Zobrazit abstrakt
While photoreceptor loss is the most devastating result of inherited retinal degenerations such as retinitis pigmentosa, inner retinal neurons also undergo significant alteration. Detailing these changes has become important as many vision restorative therapies target the remaining neurons. In this study, the rd1-Fos-Tau-LacZ (rd1-FTL) mouse model was used to explore inner retinal change at a late stage of retinal degeneration, after the loss of photoreceptor nuclei. The rd1-FTL model carries a mutation in the phosphodiesterase gene, Pde6b, and an axonally targeted transgenic beta galactosidase reporter system under the control of the c-fos promoter. Retinae of transgenic rd1-FTL mice and control FTL animals aged 2-12 months were processed for indirect fluorescence immunocytochemistry. At 2 months of age, a time when the majority of photoreceptor nuclei are lost, there was negligible c-fos reporter (FTL) expression, however, from 4 months, reporter expression was observed to increase within subpopulations of amacrine and ganglion cells within the central retina. These areas of inner retinal FTL expression coincided with regions that contained aberrant Müller cells. Specifically, these cells exhibited reduced glutamine synthetase and Kir4.1 immunolabelling, whilst showing evidence of proliferative gliosis (increased cyclinD1 and glial fibrillary acidic protein expression). These changes were limited to distinct regions where cone photoreceptor terminals were absent. Overall, these results highlight that distinct areas of the rd1-FTL central retina undergo significant glial alterations after cone photoreceptor loss. These areas coincide with up-regulation of the c-fos reporter in the inner retina, which may represent a change in neuronal function/plasticity. The rd1-FTL mouse is a useful model system to probe changes that occur in the inner retina at later stages of retinal degeneration. | | | 26283925
 |
Activity-regulated trafficking of the palmitoyl-acyl transferase DHHC5.
Brigidi, GS; Santyr, B; Shimell, J; Jovellar, B; Bamji, SX
Nature communications
6
8200
2015
Zobrazit abstrakt
Synaptic plasticity is mediated by the dynamic localization of proteins to and from synapses. This is controlled, in part, through activity-induced palmitoylation of synaptic proteins. Here we report that the ability of the palmitoyl-acyl transferase, DHHC5, to palmitoylate substrates in an activity-dependent manner is dependent on changes in its subcellular localization. Under basal conditions, DHHC5 is bound to PSD-95 and Fyn kinase, and is stabilized at the synaptic membrane through Fyn-mediated phosphorylation of a tyrosine residue within the endocytic motif of DHHC5. In contrast, DHHC5's substrate, δ-catenin, is highly localized to dendritic shafts, resulting in the segregation of the enzyme/substrate pair. Neuronal activity disrupts DHHC5/PSD-95/Fyn kinase complexes, enhancing DHHC5 endocytosis, its translocation to dendritic shafts and its association with δ-catenin. Following DHHC5-mediated palmitoylation of δ-catenin, DHHC5 and δ-catenin are trafficked together back into spines where δ-catenin increases cadherin stabilization and recruitment of AMPA receptors to the synaptic membrane. | | | 26334723
 |
Dopamine in the auditory brainstem and midbrain: co-localization with amino acid neurotransmitters and gene expression following cochlear trauma.
Fyk-Kolodziej, BE; Shimano, T; Gafoor, D; Mirza, N; Griffith, RD; Gong, TW; Holt, AG
Frontiers in neuroanatomy
9
88
2015
Zobrazit abstrakt
Dopamine (DA) modulates the effects of amino acid neurotransmitters (AANs), including GABA and glutamate, in motor, visual, olfactory, and reward systems (Hnasko et al., 2010; Stuber et al., 2010; Hnasko and Edwards, 2012). The results suggest that DA may play a similar modulatory role in the auditory pathways. Previous studies have shown that deafness results in decreased GABA release, changes in excitatory neurotransmitter levels, and increased spontaneous neuronal activity within brainstem regions related to auditory function. Modulation of the expression and localization of tyrosine hydroxylase (TH; the rate limiting enzyme in the production of DA) in the IC following cochlear trauma has been previously reported (Tong et al., 2005). In the current study the possibility of co-localization of TH with AANs was examined. Changes in the gene expression of TH were compared with changes in the gene expression of markers for AANs in the cochlear nucleus (CN) and inferior colliculus (IC) to determine whether those deafness related changes occur concurrently. The results indicate that bilateral cochlear ablation significantly reduced TH gene expression in the CN after 2 months while in the IC the reduction in TH was observed at both 3 days and 2 months following ablation. Furthermore, in the CN, glycine transporter 2 (GLYT2) and the GABA transporter (GABAtp) were also significantly reduced only after 2 months. However, in the IC, DA receptor 1 (DRDA1), vesicular glutamate transporters 2 and 3 (VGLUT2, VGLUT3), GABAtp and GAD67 were reduced in expression both at the 3 days and 2 months time points. A close relationship between the distribution of TH and several of the AANs was determined in both the CN and the IC. In addition, GLYT2 and VGLUT3 each co-localized with TH within IC somata and dendrites. Therefore, the results of the current study suggest that DA is spatially well positioned to influence the effects of AANs on auditory neurons. | | | 26257610
 |
Regulation of density of functional presynaptic terminals by local energy supply.
Zhou, H; Liu, G
Molecular brain
8
42
2015
Zobrazit abstrakt
The density of functional synapses is an important parameter in determining the efficacy of synaptic transmission. However, how functional presynaptic terminal density is regulated under natural physiological conditions is still poorly understood.We studied the factors controlling the density of presynaptic functional terminals at single dendritic branches of hippocampal neurons and found that elevation of intracellular Mg(2+) concentration was effective in increasing the density of functional terminals. Interestingly, the upregulation was not due to synaptogenesis, but to the conversion of a considerable proportion of presynaptic terminals from nonfunctional to functional. Mechanistic studies revealed that the nonfunctional terminals had inadequate Ca(2+)-sensitivity-related proteins, resulting in very low Ca(2+) sensitivity within their vesicle release machinery. We identified energy-dependent axonal transport as a primary factor controlling the amount of Ca(2+)-sensitivity-related proteins in terminals. The elevation of intracellular Mg(2+) enhanced local energy supply and promoted the increase of Ca(2+)-sensitivity-related proteins in terminals, leading to increased functional terminal density.Our study suggests that local energy supply plays a critical role in controlling the density of functional presynaptic terminals, demonstrating the link between energy supply and efficacy of synaptic transmission. | | | 26184109
 |
Aberrant synaptic integration in adult lamina I projection neurons following neonatal tissue damage.
Li, J; Kritzer, E; Craig, PE; Baccei, ML
The Journal of neuroscience : the official journal of the Society for Neuroscience
35
2438-51
2015
Zobrazit abstrakt
Mounting evidence suggests that neonatal tissue damage evokes alterations in spinal pain reflexes which persist into adulthood. However, less is known about potential concomitant effects on the transmission of nociceptive information to the brain, as the degree to which early injury modulates synaptic integration and membrane excitability in mature spinal projection neurons remains unclear. Here we demonstrate that neonatal surgical injury leads to a significant shift in the balance between synaptic excitation and inhibition onto identified lamina I projection neurons of the adult mouse spinal cord. The strength of direct primary afferent input to mature spino-parabrachial neurons was enhanced following neonatal tissue damage, whereas the efficacy of both GABAergic and glycinergic inhibition onto the same population was compromised. This was accompanied by reorganization in the pattern of sensory input to adult projection neurons, which included a greater prevalence of monosynaptic input from low-threshold A-fibers when preceded by early tissue damage. In addition, neonatal incision resulted in greater primary afferent-evoked action potential discharge in mature projection neurons. Overall, these results demonstrate that tissue damage during early life causes a long-term increase in the gain of spinal nociceptive circuits, and suggest that the prolonged consequences of neonatal trauma may not be restricted to the spinal cord but rather include excessive ascending signaling to supraspinal pain centers. | | | 25673839
 |
Cis and trans RET signaling control the survival and central projection growth of rapidly adapting mechanoreceptors.
Fleming, MS; Vysochan, A; Paixão, S; Niu, J; Klein, R; Savitt, JM; Luo, W
eLife
4
e06828
2015
Zobrazit abstrakt
RET can be activated in cis or trans by its co-receptors and ligands in vitro, but the physiological roles of trans signaling are unclear. Rapidly adapting (RA) mechanoreceptors in dorsal root ganglia (DRGs) express Ret and the co-receptor Gfrα2 and depend on Ret for survival and central projection growth. Here, we show that Ret and Gfrα2 null mice display comparable early central projection deficits, but Gfrα2 null RA mechanoreceptors recover later. Loss of Gfrα1, the co-receptor implicated in activating RET in trans, causes no significant central projection or cell survival deficit, but Gfrα1;Gfrα2 double nulls phenocopy Ret nulls. Finally, we demonstrate that GFRα1 produced by neighboring DRG neurons activates RET in RA mechanoreceptors. Taken together, our results suggest that trans and cis RET signaling could function in the same developmental process and that the availability of both forms of activation likely enhances but not diversifies outcomes of RET signaling. | | | 25838128
 |
Mapping synapses by conjugate light-electron array tomography.
Collman, F; Buchanan, J; Phend, KD; Micheva, KD; Weinberg, RJ; Smith, SJ
The Journal of neuroscience : the official journal of the Society for Neuroscience
35
5792-807
2015
Zobrazit abstrakt
Synapses of the mammalian CNS are diverse in size, structure, molecular composition, and function. Synapses in their myriad variations are fundamental to neural circuit development, homeostasis, plasticity, and memory storage. Unfortunately, quantitative analysis and mapping of the brain's heterogeneous synapse populations has been limited by the lack of adequate single-synapse measurement methods. Electron microscopy (EM) is the definitive means to recognize and measure individual synaptic contacts, but EM has only limited abilities to measure the molecular composition of synapses. This report describes conjugate array tomography (AT), a volumetric imaging method that integrates immunofluorescence and EM imaging modalities in voxel-conjugate fashion. We illustrate the use of conjugate AT to advance the proteometric measurement of EM-validated single-synapse analysis in a study of mouse cortex. | | | 25855189
 |
Loss of F-box only protein 2 (Fbxo2) disrupts levels and localization of select NMDA receptor subunits, and promotes aberrant synaptic connectivity.
Atkin, G; Moore, S; Lu, Y; Nelson, RF; Tipper, N; Rajpal, G; Hunt, J; Tennant, W; Hell, JW; Murphy, GG; Paulson, H
The Journal of neuroscience : the official journal of the Society for Neuroscience
35
6165-78
2015
Zobrazit abstrakt
NMDA receptors (NMDARs) play an essential role in some forms of synaptic plasticity, learning, and memory. Therefore, these receptors are highly regulated with respect to their localization, activation, and abundance both within and on the surface of mammalian neurons. Fundamental questions remain, however, regarding how this complex regulation is achieved. Using cell-based models and F-box Only Protein 2 (Fbxo2) knock-out mice, we found that the ubiquitin ligase substrate adaptor protein Fbxo2, previously reported to facilitate the degradation of the NMDAR subunit GluN1 in vitro, also functions to regulate GluN1 and GluN2A subunit levels in the adult mouse brain. In contrast, GluN2B subunit levels are not affected by the loss of Fbxo2. The loss of Fbxo2 results in greater surface localization of GluN1 and GluN2A, together with increases in the synaptic markers PSD-95 and Vglut1. These synaptic changes do not manifest as neurophysiological differences or alterations in dendritic spine density in Fbxo2 knock-out mice, but result instead in increased axo-dendritic shaft synapses. Together, these findings suggest that Fbxo2 controls the abundance and localization of specific NMDAR subunits in the brain and may influence synapse formation and maintenance. | | | 25878288
 |
Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture.
Paşca, AM; Sloan, SA; Clarke, LE; Tian, Y; Makinson, CD; Huber, N; Kim, CH; Park, JY; O'Rourke, NA; Nguyen, KD; Smith, SJ; Huguenard, JR; Geschwind, DH; Barres, BA; Paşca, SP
Nature methods
12
671-8
2015
Zobrazit abstrakt
The human cerebral cortex develops through an elaborate succession of cellular events that, when disrupted, can lead to neuropsychiatric disease. The ability to reprogram somatic cells into pluripotent cells that can be differentiated in vitro provides a unique opportunity to study normal and abnormal corticogenesis. Here, we present a simple and reproducible 3D culture approach for generating a laminated cerebral cortex-like structure, named human cortical spheroids (hCSs), from pluripotent stem cells. hCSs contain neurons from both deep and superficial cortical layers and map transcriptionally to in vivo fetal development. These neurons are electrophysiologically mature, display spontaneous activity, are surrounded by nonreactive astrocytes and form functional synapses. Experiments in acute hCS slices demonstrate that cortical neurons participate in network activity and produce complex synaptic events. These 3D cultures should allow a detailed interrogation of human cortical development, function and disease, and may prove a versatile platform for generating other neuronal and glial subtypes in vitro. | | | 26005811
 |
Adolescent intermittent alcohol exposure: persistence of structural and functional hippocampal abnormalities into adulthood.
Risher, ML; Fleming, RL; Risher, WC; Miller, KM; Klein, RC; Wills, T; Acheson, SK; Moore, SD; Wilson, WA; Eroglu, C; Swartzwelder, HS
Alcoholism, clinical and experimental research
39
989-97
2015
Zobrazit abstrakt
Human adolescence is a crucial stage of neurological development during which ethanol (EtOH) consumption is often at its highest. Alcohol abuse during adolescence may render individuals at heightened risk for subsequent alcohol abuse disorders, cognitive dysfunction, or other neurological impairments by irreversibly altering long-term brain function. To test this possibility, we modeled adolescent alcohol abuse (i.e., intermittent EtOH exposure during adolescence [AIE]) in rats to determine whether adolescent exposure to alcohol leads to long-term structural and functional changes that are manifested in adult neuronal circuitry.We specifically focused on hippocampal area CA1, a brain region associated with learning and memory. Using electrophysiological, immunohistochemical, and neuroanatomical approaches, we measured post-AIE changes in synaptic plasticity, dendritic spine morphology, and synaptic structure in adulthood.We found that AIE-pretreated adult rats manifest robust long-term potentiation, induced at stimulus intensities lower than those required in controls, suggesting a state of enhanced synaptic plasticity. Moreover, AIE resulted in an increased number of dendritic spines with characteristics typical of immaturity. Immunohistochemistry-based analysis of synaptic structures indicated a significant decrease in the number of co-localized pre- and postsynaptic puncta. This decrease is driven by an overall decrease in 2 postsynaptic density proteins, PSD-95 and SAP102.Taken together, these findings reveal that repeated alcohol exposure during adolescence results in enduring structural and functional abnormalities in the hippocampus. These synaptic changes in the hippocampal circuits may help to explain learning-related behavioral changes in adult animals preexposed to AIE. | | | 25916839
 |