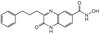
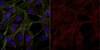
AM30 Sigma-AldrichAnti-PARP-1 (Ab-2) Mouse mAb (C-2-10)
Recommended Products
Přehled
| Replacement Information |
|---|
Tabulka spec. kláve
| Host |
|---|
| M |
| Description | |
|---|---|
| Overview | This product has been discontinued. |
| Catalogue Number | AM30 |
| Brand Family | Calbiochem® |
| Synonyms | Anti-Poly(ADP-Ribose) Polymerase |
| Application Data |  Detection of human PARP-1 by immunoblotting. Samples: Whole cell lysate from HL60 cells left untreated (lane 1) and treated with etoposide (lane 2). Primary antibody: Anti-PARP-1 (Ab-2) Mouse mAb (C-2-10) (Cat. No. AM30) (1 µg/ml). Detection: chemiluminescence. |
| Product Information | |
|---|---|
| Form | Liquid |
| Formulation | In PBS, 0.2% BSA. |
| Positive control | HL60 cells treated with etoposide |
| Preservative | ≤0.1% sodium azide |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalogové číslo | GTIN |
| AM30 | 0 |
Documentation
Anti-PARP-1 (Ab-2) Mouse mAb (C-2-10) MSDS
| Title |
|---|
Anti-PARP-1 (Ab-2) Mouse mAb (C-2-10) Certificates of Analysis
| Title | Lot Number |
|---|---|
| AM30 |
References
| Přehled odkazů |
|---|
| Leist, M., et al. 1997. Biochem. Biophys. Res. Commun. 233, 518. Posmantur, R., et al. 1997. J. Neurochem. 68, 2328. Rosen, A., and Casciola-Rosen, L. 1997. J. Cell. Biochem. 64, 50. Rosenthal, D.S., et al. 1997. Exp. Cell. Res. 232, 313. Whitacre, C.M. and Berger, N.A. 1997. Cancer Res. 57, 2157. Shah, G.M., et al. 1996. Biochem. Biophys. Res. Commun. 229,838. Yoshida, S., and Simbulan, C.M. 1994. Mol. Cell Biochem. 138, 39. Satoh, M.S., and Lindahl, T. 1992. Nature 356, 356. Lamarre, D. et al. 1988. Biochem Biophys Acta 95. Berger, N.A. 1985. Radiat. Res. 101, 4. |
Brochure
| Title |
|---|
| Caspases and other Apoptosis Related Tools Brochure |
Citations
| Název | |
|---|---|
|
|
| Data Sheet | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|