Genome-wide profiling of nardilysin target genes reveals its role in epigenetic regulation and cell cycle progression.
Morita, Y; Ohno, M; Nishi, K; Hiraoka, Y; Saijo, S; Matsuda, S; Kita, T; Kimura, T; Nishi, E
Sci Rep
7
14801
2016
Zobrazit abstrakt
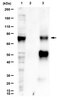
Post-translational histone modifications, such as acetylation and methylation, are prerequisites for transcriptional regulation. The metalloendopeptidase nardilysin (Nrdc) is a H3K4me2-binding protein that controls thermoregulation and β-cell functions through its transcriptional coregulator function. We herein combined high-throughput ChIP-seq and RNA-seq to achieve the first genome-wide identification of Nrdc target genes. A ChIP-seq analysis of immortalized mouse embryo fibroblasts (iMEF) identified 4053 Nrdc-binding sites, most of which were located in proximal promoter sites (2587 Nrdc-binding genes). Global H3K4me2 levels at Nrdc-binding promoters slightly increased, while H3K9ac levels decreased in the absence of Nrdc. Among Nrdc-binding genes, a comparative RNA-seq analysis identified 448 candidates for Nrdc target genes, among which cell cycle-related genes were significantly enriched. We confirmed decreased mRNA and H3K9ac levels at the promoters of individual genes in Nrdc-deficient iMEF, which were restored by the ectopic introduction of Nrdc. Reduced mRNA levels, but not H3K9ac levels were fully restored by the reintroduction of the peptidase-dead mutant of Nrdc. Furthermore, Nrdc promoted cell cycle progression at multiple stages, which enhanced cell proliferation in vivo. Collectively, our integrative studies emphasize the importance of Nrdc for maintaining a proper epigenetic status and cell growth. | 29093577
 |
Nardilysin Is Required for Maintaining Pancreatic β-Cell Function.
Nishi, K; Sato, Y; Ohno, M; Hiraoka, Y; Saijo, S; Sakamoto, J; Chen, PM; Morita, Y; Matsuda, S; Iwasaki, K; Sugizaki, K; Harada, N; Mukumoto, Y; Kiyonari, H; Furuyama, K; Kawaguchi, Y; Uemoto, S; Kita, T; Inagaki, N; Kimura, T; Nishi, E
Diabetes
65
3015-27
2015
Zobrazit abstrakt
Type 2 diabetes (T2D) is associated with pancreatic β-cell dysfunction, manifested by reduced glucose-stimulated insulin secretion (GSIS). Several transcription factors enriched in β-cells, such as MafA, control β-cell function by organizing genes involved in GSIS. Here we demonstrate that nardilysin (N-arginine dibasic convertase; Nrd1 and NRDc) critically regulates β-cell function through MafA. Nrd1(-/-) mice showed glucose intolerance and severely decreased GSIS. Islets isolated from Nrd1(-/-) mice exhibited reduced insulin content and impaired GSIS in vitro. Moreover, β-cell-specific NRDc-deficient (Nrd1(delβ)) mice showed a diabetic phenotype with markedly reduced GSIS. MafA was specifically downregulated in islets from Nrd1(delβ) mice, whereas overexpression of NRDc upregulated MafA and insulin expression in INS832/13 cells. Chromatin immunoprecipitation assay revealed that NRDc is associated with Islet-1 in the enhancer region of MafA, where NRDc controls the recruitment of Islet-1 and MafA transcription. Our findings demonstrate that NRDc controls β-cell function via regulation of the Islet-1-MafA pathway. | 27385158
 |








 Antibody[210154-ALL].jpg)
 Antibody[210154-ALL].jpg)