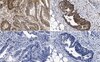
Influence of extracellular matrix components on the expression of integrins and regeneration of adult retinal ganglion cells.
Vecino, E; Heller, JP; Veiga-Crespo, P; Martin, KR; Fawcett, JW
PloS one
10
e0125250
2015
Zobrazit abstrakt
Retinal ganglion cells (RGCs) are exposed to injury in a variety of optic nerve diseases including glaucoma. However, not all cells respond in the same way to damage and the capacity of individual RGCs to survive or regenerate is variable. In order to elucidate factors that may be important for RGC survival and regeneration we have focussed on the extracellular matrix (ECM) and RGC integrin expression. Our specific questions were: (1) Do adult RGCs express particular sets of integrins in vitro and in vivo? (2) Can the nature of the ECM influence the expression of different integrins? (3) Can the nature of the ECM affect the survival of the cells and the length or branching complexity of their neurites?Primary RGC cultures from adult rat retina were placed on glass coverslips treated with different substrates: Poly-L-Lysine (PL), or PL plus laminin (L), collagen I (CI), collagen IV (CIV) or fibronectin (F). After 10 days in culture, we performed double immunostaining with an antibody against βIII-Tubulin to identify the RGCs, and antibodies against the integrin subunits: αV, α1, α3, α5, β1 or β3. The number of adhering and surviving cells, the number and length of the neurites and the expression of the integrin subunits on the different substrates were analysed.PL and L were associated with the greatest survival of RGCs while CI provided the least favourable conditions. The type of substrate affected the number and length of neurites. L stimulated the longest growth. We found at least three different types of RGCs in terms of their capacity to regenerate and extend neurites. The different combinations of integrins expressed by the cells growing on different substrata suggest that RGCs expressed predominantly α1β1 or α3β1 on L, α1β1 on CI and CIV, and α5β3 on F. The activity of the integrins was demonstrated by the phosphorylation of focal adhesion kinase (FAK).Adult rat RGCs can survive and grow in the presence of different ECM tested. Further studies should be done to elucidate the different molecular characteristics of the RGCs subtypes in order to understand the possible different sensitivity of different RGCs to damage in diseases like glaucoma in which not all RGCs die at the same time. | | | 26018803
 |
Exclusion of integrins from CNS axons is regulated by Arf6 activation and the AIS.
Franssen, EH; Zhao, RR; Koseki, H; Kanamarlapudi, V; Hoogenraad, CC; Eva, R; Fawcett, JW
The Journal of neuroscience : the official journal of the Society for Neuroscience
35
8359-75
2015
Zobrazit abstrakt
Integrins are adhesion and survival molecules involved in axon growth during CNS development, as well as axon regeneration after injury in the peripheral nervous system (PNS). Adult CNS axons do not regenerate after injury, partly due to a low intrinsic growth capacity. We have previously studied the role of integrins in axon growth in PNS axons; in the present study, we investigate whether integrin mechanisms involved in PNS regeneration may be altered or lacking from mature CNS axons by studying maturing CNS neurons in vitro. In rat cortical neurons, we find that integrins are present in axons during initial growth but later become restricted to the somato-dendritic domain. We investigated how this occurs and whether it can be altered to enhance axonal growth potential. We find a developmental change in integrin trafficking; transport becomes predominantly retrograde throughout axons, but not dendrites, as neurons mature. The directionality of transport is controlled through the activation state of ARF6, with developmental upregulation of the ARF6 GEF ARNO enhancing retrograde transport. Lowering ARF6 activity in mature neurons restores anterograde integrin flow, allows transport into axons, and increases axon growth. In addition, we found that the axon initial segment is partly responsible for exclusion of integrins and removal of this structure allows integrins into axons. Changing posttranslational modifications of tubulin with taxol also allows integrins into the proximal axon. The experiments suggest that the developmental loss of regenerative ability in CNS axons is due to exclusion of growth-related molecules due to changes in trafficking. | | | 26019348
 |
Blocking αVβ3 integrin ligand occupancy inhibits the progression of albuminuria in diabetic rats.
Maile, LA; Gollahon, K; Wai, C; Dunbar, P; Busby, W; Clemmons, D
Journal of diabetes research
2014
421827
2014
Zobrazit abstrakt
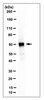
This study determined if blocking ligand occupancy of the αVβ3 integrin could inhibit the pathophysiologic changes that occur in the early stages of diabetic nephropathy (DN). Diabetic rats were treated with either vehicle or a monoclonal antibody that binds the β3 subunit of the αVβ3 integrin. After 4 weeks of diabetes the urinary albumin to creatinine ratio (UACR) increased in both diabetic animals that subsequently received vehicle and in the animals that subsequently received the anti-β3 antibody compared with control nondiabetic rats. After 8 weeks of treatment the UACR continued to rise in the vehicle-treated rats; however it returned to levels comparable to control nondiabetic rats in rats treated with the anti-β3 antibody. Treatment with the antibody prevented the increase of several profibrotic proteins that have been implicated in the development of DN. Diabetes was associated with an increase in phosphorylation of the β3 subunit in kidney homogenates from diabetic animals, but this was prevented by the antibody treatment. This study demonstrates that, when administered after establishment of early pathophysiologic changes in renal function, the anti-β3 antibody reversed the effects of diabetes normalizing albuminuria and profibrotic proteins in the kidney to the levels observed in nondiabetic control animals. | Western Blotting | | 25389530
 |
Small molecule proprotein convertase inhibitors for inhibition of embryo implantation.
Ho, H; Singh, H; Heng, S; Nero, TL; Paule, S; Parker, MW; Johnson, AT; Jiao, GS; Nie, G
PloS one
8
e81380
2013
Zobrazit abstrakt
Uterine proprotein convertase (PC) 6 plays a critical role in embryo implantation and is pivotal for pregnancy establishment. Inhibition of PC6 may provide a novel approach for the development of non-hormonal and female-controlled contraceptives. We investigated a class of five synthetic non-peptidic small molecule compounds that were previously reported as potent inhibitors of furin, another PC member. We examined (i) the potency of these compounds in inhibiting PC6 activity in vitro; (ii) their binding modes in the PC6 active site in silico; (iii) their efficacy in inhibiting PC6-dependent cellular processes essential for embryo implantation using human cell-based models. All five compounds showed potent inhibition of PC6 activity in vitro, and in silico docking demonstrated that these inhibitors could adopt a similar binding mode in the PC6 active site. However, when these compounds were tested for their inhibition of decidualization of primary human endometrial stromal cells, a PC6-dependent cellular process critical for embryo implantation, only one (compound 1o) showed potent inhibition. The lack of activity in the cell-based assay may reflect the inability of the compounds to penetrate the cell membrane. Because compound's lipophilicity is linked to cell penetration, a measurement of lipophilicity (logP) was calculated for each compound. Compound 1o is unique as it appears the most lipophilic among the five compounds. Compound 1o also inhibited another crucial PC6-dependent process, the attachment of human trophoblast spheroids to endometrial epithelial cells (a model for human embryo attachment). We thus identified compound 1o as a potent small molecule PC6 inhibitor with pharmaceutical potential to inhibit embryo implantation. Our findings also highlight that human cell-based functional models are vital to complement the biochemical and in silico analyses in the selection of promising drug candidates. Further investigations for compound 1o are warranted in animal models to test its utility as an implantation-inhibiting contraceptive drug. | | | 24324690
 |
Cyp1b1 mediates periostin regulation of trabecular meshwork development by suppression of oxidative stress.
Zhao, Y; Wang, S; Sorenson, CM; Teixeira, L; Dubielzig, RR; Peters, DM; Conway, SJ; Jefcoate, CR; Sheibani, N
Molecular and cellular biology
33
4225-40
2013
Zobrazit abstrakt
Mutation in CYP1B1 has been reported for patients with congenital glaucoma. However, the underlying mechanisms remain unknown. Here we show increased diurnal intraocular pressure (IOP) in Cyp1b1-deficient (Cyp1b1(-/-)) mice. Cyp1b1(-/-) mice presented ultrastructural irregular collagen distribution in their trabecular meshwork (TM) tissue along with increased oxidative stress and decreased levels of periostin (Postn). Increased levels of oxidative stress and decreased levels of Postn were also detected in human glaucomatous TM tissues. Furthermore, Postn-deficient mice exhibited TM tissue ultrastructural abnormalities similar to those of Cyp1b1(-/-) mice. Administration of the antioxidant N-acetylcysteine (NAC) restored structural abnormality of TM tissue in Cyp1b1(-/-) mice. In addition, TM cells prepared from Cyp1b1(-/-) mice exhibited increased oxidative stress, altered adhesion, and decreased levels of Postn. These aberrant cellular responses were reversed in the presence of NAC or by restoration of Cyp1b1 expression. Cyp1b1 knockdown or inhibition of CYP1B1 activity in Cyp1b1(+/+) TM cells resulted in a Cyp1b1(-/-) phenotype. Thus, metabolic activity of CYP1B1 contributes to oxidative homeostasis and ultrastructural organization and function of TM tissue through modulation of Postn expression. | Dot Blot | Mouse | 23979599
 |
Integrin upregulation and localization to focal adhesion sites in pregnant human myometrium.
Burkin, HR; Rice, M; Sarathy, A; Thompson, S; Singer, CA; Buxton, IL
Reproductive sciences (Thousand Oaks, Calif.)
20
804-12
2013
Zobrazit abstrakt
Focal adhesions are integrin-rich microdomains that structurally link the cytoskeleton to the extracellular matrix and transmit mechanical signals. In the pregnant uterus, increases in integrin expression and activation are thought to be critical for the formation of the mechanical syncytium required for labor. The aim of this study was to determine which integrins are upregulated and localized to focal adhesions in pregnant human myometrium. We used quantitative polymerase chain reaction, Western blotting, and confocal microscopy to determine the expression levels and colocalization with focal adhesion proteins. We observed increases in several integrin transcripts in pregnant myometrium. At the protein level, integrins such as α5-integrin (ITGA5), ITGA7, ITGAV, and ITGB3 were significantly increased during pregnancy. The integrins ITGA3, ITGA5, ITGA7, and ITGB1 colocalized with focal adhesion proteins in term human myometrium. These data suggest that integrins α3β1, α5β1, and α7β1 are the most likely candidates to transmit mechanical signals from the extracellular matrix through focal adhesions in pregnant human myometrium. | | | 23298868
 |
Cleavage of endometrial α-integrins into their functional forms is mediated by proprotein convertase 5/6.
Paule, S; Aljofan, M; Simon, C; Rombauts, LJ; Nie, G
Human reproduction (Oxford, England)
27
2766-74
2011
Zobrazit abstrakt
Proprotein convertases (PCs) post-translationally activate a large number of protein precursors through limited cleavage. PC5/6 (PC6) in the human endometrium is tightly regulated during receptivity for embryo implantation. Integrins are transmembrane glycoproteins, some of which play an important role in the adhesive interactions between the trophoblast (blastocyst) and uterine epithelium at implantation. Integrins require PC cleavage for post-translational modification. We hypothesize that pro-integrin-αs in the endometrial epithelium are post-translationally cleaved by PC6 into functional subunits for the binding of blastocyst and adhesion of extracellular matrix proteins.We first used the endometrial epithelial cell line, HEC1A, into which siRNA specific to human PC6 (PC6-siRNA) or scrambled sequence (control) was stably transfected. The specific knockdown was confirmed by real-time RT-PCR. PC6-siRNA cells reduced their capacity to attach to trophoblast spheroids and bind to fibronectin compared with control. Knockdown of PC6 decreased cell surface presentation of functional integrins-α1, α2, α5, αV and αVβ5. Western blot analysis demonstrated that PC6 was responsible for the post-translational cleavage of pro-integrin-α5 and integrin-αV into their heavy and light chains in HEC1A cells. We then isolated primary human endometrial epithelial cells and validated that PC6 mediated the post-translational cleavage of integrin-αs in these cells.This study implicates PC6 as a key regulatory protein essential for the attachment of the blastocyst to the endometrial epithelium through the processing of pro-integrin-αs. Compromised PC6 action reduces the post-translational modification of integrin-αs, thus compromising implantation. | | | 22740495
 |
A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis.
Reticker-Flynn, NE; Malta, DF; Winslow, MM; Lamar, JM; Xu, MJ; Underhill, GH; Hynes, RO; Jacks, TE; Bhatia, SN
Nature communications
3
1122
2011
Zobrazit abstrakt
Extracellular matrix interactions have essential roles in normal physiology and many pathological processes. Although the importance of extracellular matrix interactions in metastasis is well documented, systematic approaches to identify their roles in distinct stages of tumorigenesis have not been described. Here we report a novel-screening platform capable of measuring phenotypic responses to combinations of extracellular matrix molecules. Using a genetic mouse model of lung adenocarcinoma, we measure the extracellular matrix-dependent adhesion of tumour-derived cells. Hierarchical clustering of the adhesion profiles differentiates metastatic cell lines from primary tumour lines. Furthermore, we uncovered that metastatic cells selectively associate with fibronectin when in combination with galectin-3, galectin-8 or laminin. We show that these molecules correlate with human disease and that their interactions are mediated in part by α3β1 integrin. Thus, our platform allowed us to interrogate interactions between metastatic cells and their microenvironments, and identified extracellular matrix and integrin interactions that could serve as therapeutic targets. | Immunohistochemistry | | 23047680
 |
SPARC promotes pericyte recruitment via inhibition of endoglin-dependent TGF-β1 activity.
Rivera, LB; Brekken, RA
The Journal of cell biology
193
1305-19
2010
Zobrazit abstrakt
Pericytes migrate to nascent vessels and promote vessel stability. Recently, we reported that secreted protein acidic and rich in cysteine (SPARC)-deficient mice exhibited decreased pericyte-associated vessels in an orthotopic model of pancreatic cancer, suggesting that SPARC influences pericyte behavior. In this paper, we report that SPARC promotes pericyte migration by regulating the function of endoglin, a TGF-β1 accessory receptor. Primary SPARC-deficient pericytes exhibited increased basal TGF-β1 activity and decreased cell migration, an effect blocked by inhibiting TGF-β1. Furthermore, TGF-β-mediated inhibition of pericyte migration was dependent on endoglin and αV integrin. SPARC interacted directly with endoglin and reduced endoglin interaction with αV integrin. SPARC deficiency resulted in endoglin-mediated blockade of pericyte migration, aberrant association of endoglin in focal complexes, an increase in αV integrins present in endoglin immunoprecipitates, and enhanced αV integrin-mediated activation of TGF-β. These results demonstrate that SPARC promotes pericyte migration by diminishing TGF-β activity and identify a novel function for endoglin in controlling pericyte behavior. | | | 21708981
 |
Endothelial von Willebrand factor regulates angiogenesis.
Starke, RD; Ferraro, F; Paschalaki, KE; Dryden, NH; McKinnon, TA; Sutton, RE; Payne, EM; Haskard, DO; Hughes, AD; Cutler, DF; Laffan, MA; Randi, AM
Blood
117
1071-80
2010
Zobrazit abstrakt
The regulation of blood vessel formation is of fundamental importance to many physiological processes, and angiogenesis is a major area for novel therapeutic approaches to diseases from ischemia to cancer. A poorly understood clinical manifestation of pathological angiogenesis is angiodysplasia, vascular malformations that cause severe gastrointestinal bleeding. Angiodysplasia can be associated with von Willebrand disease (VWD), the most common bleeding disorder in man. VWD is caused by a defect or deficiency in von Willebrand factor (VWF), a glycoprotein essential for normal hemostasis that is involved in inflammation. We hypothesized that VWF regulates angiogenesis. Inhibition of VWF expression by short interfering RNA (siRNA) in endothelial cells (ECs) caused increased in vitro angiogenesis and increased vascular endothelial growth factor (VEGF) receptor-2 (VEGFR-2)-dependent proliferation and migration, coupled to decreased integrin αvβ3 levels and increased angiopoietin (Ang)-2 release. ECs expanded from blood-derived endothelial progenitor cells of VWD patients confirmed these results. Finally, 2 different approaches, in situ and in vivo, showed increased vascularization in VWF-deficient mice. We therefore identify a new function of VWF in ECs, which confirms VWF as a protein with multiple vascular roles and defines a novel link between hemostasis and angiogenesis. These results may have important consequences for the management of VWD, with potential therapeutic implications for vascular diseases. | | | 21048155
 |