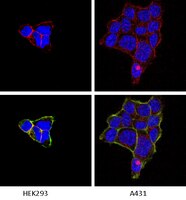
Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop
Daniel Öhlund 1 , Oskar Franklin, Erik Lundberg, Christina Lundin, Malin Sund
BMC Cancer
13
154
2013
Zobrazit abstrakt
Background: Pancreatic cancer shows a highly aggressive and infiltrative growth pattern and is characterized by an abundant tumor stroma known to interact with the cancer cells, and to influence tumor growth and drug resistance. Cancer cells actively take part in the production of extracellular matrix proteins, which then become deposited into the tumor stroma. Type IV collagen, an important component of the basement membrane, is highly expressed by pancreatic cancer cells both in vivo and in vitro. In this study, the cellular effects of type IV collagen produced by the cancer cells were characterized. <br />Methods: The expression of type IV collagen and its integrin receptors were examined in vivo in human pancreatic cancer tissue. The cellular effects of type IV collagen were studied in pancreatic cancer cell lines by reducing type IV collagen expression through RNA interference and by functional receptor blocking of integrins and their binding-sites on the type IV collagen molecule. <br />Results: We show that type IV collagen is expressed close to the cancer cells in vivo, forming basement membrane like structures on the cancer cell surface that colocalize with the integrin receptors. Furthermore, the interaction between type IV collagen produced by the cancer cell, and integrins on the surface of the cancer cells, are important for continuous cancer cell growth, maintenance of a migratory phenotype, and for avoiding apoptosis. <br />Conclusion: We show that type IV collagen provides essential cell survival signals to the pancreatic cancer cells through an autocrine loop. | | | 23530721
 |
Phosphatase of regenerating liver-3 directly interacts with integrin β1 and regulates its phosphorylation at tyrosine 783.
Tian, W; Qu, L; Meng, L; Liu, C; Wu, J; Shou, C
BMC biochemistry
13
22
2011
Zobrazit abstrakt
Phosphatase of regenerating liver-3 (PRL-3 or PTP4A3) has been implicated in controlling cancer cell proliferation, motility, metastasis, and angiogenesis. Deregulated expression of PRL-3 is highly correlated with cancer progression and predicts poor survival. Although PRL-3 was categorized as a tyrosine phosphatase, its cellular substrates remain largely unknown.We demonstrated that PRL-3 interacts with integrin β1 in cancer cells. Recombinant PRL-3 associates with the intracellular domain of integrin β1 in vitro. Silencing of integrin α1 enhances PRL-3-integrin β1 interaction. Furthermore, PRL-3 diminishes tyrosine phosphorylation of integrin β1 in vitro and in vivo. With site-specific anti-phosphotyrosine antibodies against residues in the intracellular domain of integrin β1, tyrosine-783, but not tyrosine-795, is shown to be dephosphorylated by PRL-3 in a catalytic activity-dependant manner. Phosphorylation of Y783 is potentiated by ablation of PRL-3 or by treatment with a chemical inhibitor of PRL-3. Conversely, depletion of integrin α1 decreases the phosphorylation of this site.Our results revealed a direct interaction between PRL-3 and integrin β1 and characterized Y783 of integrin β1 as a bona fide substrate of PRL-3, which is negatively regulated by integrin α1. | | | 23092334
 |
Integrin alpha1beta1 regulates epidermal growth factor receptor activation by controlling peroxisome proliferator-activated receptor gamma-dependent caveolin-1 expression.
Chen, X; Whiting, C; Borza, C; Hu, W; Mont, S; Bulus, N; Zhang, MZ; Harris, RC; Zent, R; Pozzi, A
Molecular and cellular biology
30
3048-58
2009
Zobrazit abstrakt
Integrin alpha1beta1 negatively regulates the generation of profibrotic reactive oxygen species (ROS) by inhibiting epidermal growth factor receptor (EGFR) activation; however, the mechanism by which it does this is unknown. In this study, we show that caveolin-1 (Cav-1), a scaffolding protein that binds integrins and controls growth factor receptor signaling, participates in integrin alpha1beta1-mediated EGFR activation. Integrin alpha1-null mesangial cells (MCs) have reduced Cav-1 levels, and reexpression of the integrin alpha1 subunit increases Cav-1 levels, decreases EGFR activation, and reduces ROS production. Downregulation of Cav-1 in wild-type MCs increases EGFR phosphorylation and ROS synthesis, while overexpression of Cav-1 in the integrin alpha1-null MCs decreases EGFR-mediated ROS production. We further show that integrin alpha1-null MCs have increased levels of activated extracellular signal-regulated kinase (ERK), which leads to reduced activation of peroxisome proliferator-activated receptor gamma (PPARgamma), a transcription factor that positively regulates Cav-1 expression. Moreover, activation of PPARgamma or inhibition of ERK increases Cav-1 levels in the integrin alpha1-null MCs. Finally, we show that glomeruli of integrin alpha1-null mice have reduced levels of Cav-1 and activated PPARgamma but increased levels of phosphorylated EGFR both at baseline and following injury. Thus, integrin alpha1beta1 negatively regulates EGFR activation by positively controlling Cav-1 levels, and the ERK/PPARgamma axis plays a key role in regulating integrin alpha1beta1-dependent Cav-1 expression and consequent EGFR-mediated ROS production. Celý text článku | | | 20368353
 |
Different assembly of type IV collagen on hydrophilic and hydrophobic substrata alters endothelial cells interaction.
N Miranda Coelho,C González-García,J A Planell,M Salmerón-Sánchez,G Altankov
European cells & materials
19
2009
Zobrazit abstrakt
Considering the structural role of type IV collagen (Col IV) in the assembly of the basement membrane (BM) and the perspective of mimicking its organization for vascular tissue engineering purposes, we studied the adsorption pattern of this protein on model hydrophilic (clean glass) and hydrophobic trichloro(octadecyl)silane (ODS) surfaces known to strongly affect the behavior of other matrix proteins. The amount of fluorescently labeled Col IV was quantified showing saturation of the surface for concentration of the adsorbing solution of about 50microg/ml, but with approximately twice more adsorbed protein on ODS. AFM studies revealed a fine - nearly single molecular size - network arrangement of Col IV on hydrophilic glass, which turns into a prominent and growing polygonal network consisting of molecular aggregates on hydrophobic ODS. The protein layer forms within minutes in a concentration-dependent manner. We further found that human umbilical vein endothelial cells (HUVEC) attach less efficiently to the aggregated Col IV (on ODS), as judged by the significantly altered cell spreading, focal adhesions formation and the development of actin cytoskeleton. Conversely, the immunofluorescence studies for integrins revealed that the fine Col IV network formed on hydrophilic substrata is better recognized by the cells via both alpha1 and alpha2 heterodimers which support cellular interaction, apart from these on hydrophobic ODS where almost no clustering of integrins was observed. | | | 20533192
 |
Stimulated single-cell force spectroscopy to quantify cell adhesion receptor crosstalk.
Jens Friedrichs,Jonne Helenius,Daniel J Müller
Proteomics
10
2009
Zobrazit abstrakt
To control their attachment to substrates and other cells, cells regulate their adhesion receptors. One regulatory process is receptor crosstalk, where the binding of one type of cell adhesion molecule influences the activity of another type. To identify such crosstalk and gain insight into their mechanisms, we developed the stimulated single-cell force spectroscopy assay. In this assay, the influence of a cells adhesion to one substrate on the strength of its adhesion to a second substrate is examined. The assay quantifies the adhesion of the cell and the contributions of specific adhesion receptors. This allows mechanisms by which the adhesion is regulated to be determined. Using the assay we identified crosstalk between collagen-binding integrin alpha(1)beta(1) and fibronectin-binding integrin alpha(5)beta(1) in HeLa cells. This crosstalk was unidirectional, from integrin alpha(1)beta(1) to integrin alpha(5)beta(1), and functioned by regulating the endocytosis of integrin alpha(5)beta(1). The single-cell assay should be expandable for the screening and quantification of crosstalk between various cell adhesion molecules and other cell surface receptors. | | | 20127696
 |
A role for pericytes as microenvironmental regulators of human skin tissue regeneration.
Paquet-Fifield, S; Schlüter, H; Li, A; Aitken, T; Gangatirkar, P; Blashki, D; Koelmeyer, R; Pouliot, N; Palatsides, M; Ellis, S; Brouard, N; Zannettino, A; Saunders, N; Thompson, N; Li, J; Kaur, P
The Journal of clinical investigation
119
2795-806
2009
Zobrazit abstrakt
The cellular and molecular microenvironment of epithelial stem and progenitor cells is poorly characterized despite well-documented roles in homeostatic tissue renewal, wound healing, and cancer progression. Here, we demonstrate that, in organotypic cocultures, dermal pericytes substantially enhanced the intrinsically low tissue-regenerative capacity of human epidermal cells that have committed to differentiate and that this enhancement was independent of angiogenesis. We used microarray analysis to identify genes expressed by human dermal pericytes that could potentially promote epidermal regeneration. Using this approach, we identified as a candidate the gene LAMA5, which encodes laminin alpha5, a subunit of the ECM component laminin-511/521 (LM-511/521). LAMA5 was of particular interest as we had previously shown that it promotes skin regeneration both in vitro and in vivo. Analysis using immunogold localization revealed that pericytes synthesized and secreted LAMA5 in human skin. Consistent with this observation, coculture with pericytes enhanced LM-511/521 deposition in the dermal-epidermal junction of organotypic cultures. We further showed that skin pericytes could also act as mesenchymal stem cells, exhibiting the capacity to differentiate into bone, fat, and cartilage lineages in vitro. This study suggests that pericytes represent a potent stem cell population in the skin that is capable of modifying the ECM microenvironment and promoting epidermal tissue renewal from non-stem cells, a previously unsuspected role for pericytes. | | | 19652362
 |
Defects in cell spreading and ERK12 activation in fibroblasts with lamin AC mutations.
LJ Emerson, MR Holt, MA Wheeler, M Wehnert, M Parsons, JA Ellis
Biochimica et biophysica acta
1792
810-821
2009
Zobrazit abstrakt
In-frame mutations in nuclear lamin A/C lead to a multitude of tissue-specific degenerative diseases known as the 'laminopathies'. Previous studies have demonstrated that lamin A/C-null mouse fibroblasts have defects in cell polarisation, suggesting a role for lamin A/C in nucleo-cytoskeletal-cell surface cross-talk. However, this has not been examined in patient fibroblasts expressing modified forms of lamin A/C. Here, we analysed skin fibroblasts from 3 patients with Emery-Dreifuss muscular dystrophy and from 1 with dilated cardiomyopathy. The emerin-lamin A/C interaction was impaired in each mutant cell line. Mutant cells exhibited enhanced cell proliferation, collagen-dependent adhesion, larger numbers of filopodia and smaller cell spread size, compared with control cells. Furthermore, cell migration, speed and polarization were elevated. Mutant cells also showed an enhanced ability to contract collagen gels at early time points, compared with control cells. Phosphotyrosine measurements during cell spreading indicated an initial temporal lag in ERK1/2 activation in our mutant cells, followed by hyper-activation of ERK1/2 at 2 h post cell attachment. Deregulated ERK1/2 activation is linked with cardiomyopathy, cell spreading and proliferation defects. We conclude that a functional emerin-lamin A/C complex is required for cell spreading and proliferation, possibly acting through ERK1/2 signalling., | | | 19524666
 |
Role of integrins in the assembly and function of hensin in intercalated cells.
Vijayakumar, S; Erdjument-Bromage, H; Tempst, P; Al-Awqati, Q
Journal of the American Society of Nephrology : JASN
19
1079-91
2008
Zobrazit abstrakt
Epithelial differentiation proceeds in at least two steps: Conversion of a nonepithelial cell into an epithelial sheet followed by terminal differentiation into the mature epithelial phenotype. It was recently discovered that the extracellular matrix (ECM) protein hensin is able to convert a renal intercalated cell line from a flat, squamous shape into a cuboidal or columnar epithelium. Global knockout of hensin in mice results in embryonic lethality at the time that the first columnar cells appear. Here, antibodies that either activate or block integrin beta1 were used to demonstrate that activation of integrin alpha v beta 1 causes deposition of hensin in the ECM. Once hensin polymerizes and deposits into the ECM, it binds to integrin alpha 6 and mediates the conversion of epithelial cells to a cuboidal phenotype capable of apical endocytosis; therefore, multiple integrins play a role in the terminal differentiation of the intercalated cell: alpha v beta 1 generates polymerized hensin, and another set of integrins (containing alpha 6) mediates signals between hensin and the interior of the cells. Celý text článku | Western Blotting, Immunoprecipitation | Rabbit | 18337486
 |
Progressive morphological and functional defects in retinas from alpha1 integrin-null mice.
Peng, YW; Zallocchi, M; Meehan, DT; Delimont, D; Chang, B; Hawes, N; Wang, W; Cosgrove, D
Investigative ophthalmology & visual science
49
4647-54
2008
Zobrazit abstrakt
The role of integrin/cell matrix interactions between the RPE and the basement membrane in retinal maintenance and function is not well characterized. In this study the functional importance of alpha1beta1 integrin for retinal pigment epithelial cell homeostasis and retinal health was assessed by comparing alpha1 integrin knockout mice with strain- and age-matched wild-type mice.Immunolocalization and Western blot analysis of retinas and ARPE19 cells were performed to examine the expression of alpha1beta1 integrin in the RPE. Retinal abnormality was assessed by funduscopy, histology, and transmission electron microscopy. Progressive retinal damage was quantified by direct counting of rod photoreceptors. Light-induced translocation of arrestin and alpha-transducin was documented by immunohistochemical analysis of retinal cryosections.Integrin alpha1beta1 localizes to the basal aspect of retinal pigment epithelial cells colocalizing with the basal lamina of the RPE. Integrin alpha1-null mice have delayed-onset progressive retinal degeneration associated with thickening of the basement membrane, dysmorphology of basal processes, synaptic malformations, and funduscopic abnormalities. Integrin alpha1-null mice display marked delays in transducin translocation compared with dark-adapted wild-type mice after exposure to light.Collectively, these data suggest an essential role for alpha1beta1 integrin/basement membrane interactions in the RPE in basement membrane metabolism and translocation of transducin in photoreceptors. This is the first report describing evidence supporting an essential role for integrin/basement membrane interaction in the RPE. Further, this report demonstrates a direct link between integrin alpha1beta1 function in retinal pigment epithelial and molecular defects in photoreceptor cell function before retinal abnormality is apparent. Celý text článku | | | 18614805
 |
Functional analysis of the cytoplasmic domain of the integrin {alpha}1 subunit in endothelial cells.
Abair, TD; Bulus, N; Borza, C; Sundaramoorthy, M; Zent, R; Pozzi, A
Blood
112
3242-54
2008
Zobrazit abstrakt
Integrin alpha1beta1, the major collagen type IV receptor, is expressed by endothelial cells and plays a role in both physiologic and pathologic angiogenesis. Because the molecular mechanisms whereby this collagen IV receptor mediates endothelial cell functions are poorly understood, truncation and point mutants of the integrin alpha1 subunit cytoplasmic tail (amino acids 1137-1151) were generated and expressed into alpha1-null endothelial cells. We show that alpha1-null endothelial cells expressing the alpha1 subunit, which lacks the entire cytoplasmic tail (mutant alpha1-1136) or expresses all the amino acids up to the highly conserved GFFKR motif (mutant alpha1-1143), have a similar phenotype to parental alpha1-null cells. Pro(1144) and Leu(1145) were shown to be necessary for alpha1beta1-mediated endothelial cell proliferation; Lys(1146) for adhesion, migration, and tubulogenesis and Lys(1147) for tubulogenesis. Integrin alpha1beta1-dependent endothelial cell proliferation is primarily mediated by ERK activation, whereas migration and tubulogenesis require both p38 MAPK and PI3K/Akt activation. Thus, distinct amino acids distal to the GFFKR motif of the alpha1 integrin cytoplasmic tail mediate activation of selective downstream signaling pathways and specific endothelial cell functions. Celý text článku | | | 18647959
 |