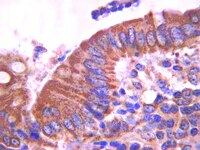
Androgen receptor-independent function of FoxA1 in prostate cancer metastasis.
Jin, HJ; Zhao, JC; Ogden, I; Bergan, RC; Yu, J
Cancer research
73
3725-36
2013
Zobrazit abstrakt
FoxA1 (FOXA1) is a pioneering transcription factor of the androgen receptor (AR) that is indispensible for the lineage-specific gene expression of the prostate. To date, there have been conflicting reports on the role of FoxA1 in prostate cancer progression and prognosis. With recent discoveries of recurrent FoxA1 mutations in human prostate tumors, comprehensive understanding of FoxA1 function has become very important. Here, through genomic analysis, we reveal that FoxA1 regulates two distinct oncogenic processes via disparate mechanisms. FoxA1 induces cell growth requiring the AR pathway. On the other hand, FoxA1 inhibits cell motility and epithelial-to-mesenchymal transition (EMT) through AR-independent mechanism directly opposing the action of AR signaling. Using orthotopic mouse models, we further show that FoxA1 inhibits prostate tumor metastasis in vivo. Concordant with these contradictory effects on tumor progression, FoxA1 expression is slightly upregulated in localized prostate cancer wherein cell proliferation is the main feature, but is remarkably downregulated when the disease progresses to metastatic stage for which cell motility and EMT are essential. Importantly, recently identified FoxA1 mutants have drastically attenuated ability in suppressing cell motility. Taken together, our findings illustrate an AR-independent function of FoxA1 as a metastasis inhibitor and provide a mechanism by which recurrent FoxA1 mutations contribute to prostate cancer progression. | | 23539448
 |
Silencing of E7 oncogene restores functional E-cadherin expression in human papillomavirus 16-transformed keratinocytes.
Caberg, Jean-Hubert D, et al.
Carcinogenesis, 29: 1441-7 (2008)
2008
Zobrazit abstrakt
Human papillomavirus (HPV) infection, particularly type 16, is causally associated with cancer of the uterine cervix. The persistence or progression of cervical lesions suggests that viral antigens are not adequately presented to the immune system. This hypothesis is reinforced by the observation that most squamous intra-epithelial lesions show quantitative and functional alterations of Langerhans cells (LCs). Moreover, E-cadherin-dependent adhesion of LC to keratinocytes (KCs) is defective in cervical HPV16-associated (pre)neoplastic lesions. The possible role of viral oncoprotein E7 in the reduced levels of cell surface E-cadherin was investigated by silencing HPV16 E7 by RNA interference (siRNA). This treatment induced an increased cell surface E-cadherin expression in HPV16-positive KC and a significant adhesion of LC to these squamous cells. The E-cadherin re-expression following HPV16 E7 silencing was associated with increased detection levels of retinoblastoma protein and the activating protein (AP)-2alpha transcription factor. These data suggest that HPV16 E7-induced alterations of LC/KC adhesion may play a role in the defective immune response during cervical carcinogenesis. | | 18566017
 |
Controlled, scalable embryonic stem cell differentiation culture.
Dang, Stephen M, et al.
Stem Cells, 22: 275-82 (2004)
2004
Zobrazit abstrakt
Embryonic stem (ES) cells are of significant interest as a renewable source of therapeutically useful cells. ES cell aggregation is important for both human and mouse embryoid body (EB) formation and the subsequent generation of ES cell derivatives. Aggregation between EBs (agglomeration), however, inhibits cell growth and differentiation in stirred or high-cell-density static cultures. We demonstrate that the agglomeration of two EBs is initiated by E-cadherin-mediated cell attachment and followed by active cell migration. We report the development of a technology capable of controlling cell-cell interactions in scalable culture by the mass encapsulation of ES cells in size-specified agarose capsules. When placed in stirred-suspension bioreactors, encapsulated ES cells can be used to produce scalable quantities of hematopoietic progenitor cells in a controlled environment. | | 15153605
 |
E-cadherin-mediated interactions of thymic epithelial cells with CD103+ thymocytes lead to enhanced thymocyte cell proliferation.
Kutlesa, Snjezana, et al.
J. Cell. Sci., 115: 4505-15 (2002)
2002
Zobrazit abstrakt
Cadherins are a family of cell adhesion molecules that mainly mediate homotypic homophilic interactions, but for E-cadherin, heterophilic interactions with the integrin alpha(E)(CD103)beta(7) have also been reported. In the human thymus, where thymocytes develop in close contact with thymic stromal cells, E-cadherin expression was detected on thymic epithelial cells. By immunofluorescence staining, the strongest expression of E-cadherin was observed on medullary thymic epithelial cells. These cells also express cytosolic catenins, which are necessary to form functional cadherin-catenin complexes. Regardless of their developmental stage, human thymocytes do not express E-cadherin, indicating that homophilic interactions cannot occur. Flow cytometric analysis revealed that the E-cadherin ligand CD103 is expressed on subpopulations of the early CD4(-) CD8(-) double-negative and of the more mature CD8(+) single-positive thymocytes. Using an in vitro cell adhesion assay, double-negative and CD8(+) single-positive thymocytes adhered strongly to isolated thymic epithelial cells. These adhesive interactions could be inhibited by antibodies against E-cadherin or CD103. CD8(+) thymocytes showed a proliferative response when incubated with thymic epithelial cells. This mitogenic effect was inhibited by antibodies against CD103, which strongly indicates a direct involvement of the adhesive ligand pair CD103-E-cadherin in human thymocyte cell proliferation. | Immunoblotting (Western) | 12414996
 |
The adhesion molecule E-cadherin and a surface antigen recognized by the antibody 9C4 are selectively expressed on erythroid cells of defined maturational stages.
Bühring, H J, et al.
Leukemia, 10: 106-16 (1996)
1996
Zobrazit abstrakt
The antigen expression of immature erythroid bone marrow cells was studied using two recently generated monoclonal antibodies (mAb), mAb 67A4 and 9C4, with specificities for the epithelial cell adhesion molecule E-cadherin (E-cad; mAb 67A4), and a novel 110 kDa differentiation antigen (mAb 9C4) with unknown molecular structure. Pappenheim staining of FACS-purified cells labeled with mAb 9C4 and anti-glycophorin A (GA) revealed that the majority of the 9C4+GA- and 9C4+GA+ cells consisted of erythroblasts. In contrast, the E-cad-positive population comprised normoblasts and erythroblasts. While the E-cad+GA- fraction contained mainly erythroblasts and basophilic normoblasts, the E-Cad+GA+ population was enriched in orthochromatic and polychromatophilic normoblasts. By colony assays of affinity column-purified cells it could be shown that erythroid colony forming units (CFU-E) were enriched and erythroid burst forming units (BFU-E) were depleted in the 9C4- and E-cad-positive fractions. Flow cytometric analysis of bone marrow cells double-labeled with mAb 67A4 and anti-CD71, anti-CD117, anti-CD34, or anti-GA revealed that about 90% of the E-cad-positive cells coexpressed CD71, about 70% were positive for CD117, about 50% for GA, and only about 5% coexpressed CD34. The expression pattern of 9C4 antigen was similar to that of E-Cad with the exception that only a minority of the 9C4-positive cells coexpressed GA. Lymphoid and myeloid markers were negative on both the E-Cad- and 9C4-positive populations. In these studies we describe the identification of a new mAb-defined antigen which is specifically expressed on erythroblasts and CFU-E(9C4) and demonstrate that E-Cad is not only expressed on epithelial cells but also on erythropoietic cells of defined maturational stages. | | 8558914
 |