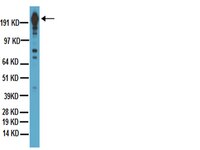
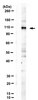
Multiple functional domains and complexes of the two nonstructural proteins of human respiratory syncytial virus contribute to interferon suppression and cellular location.
Swedan, S; Andrews, J; Majumdar, T; Musiyenko, A; Barik, S
Journal of virology
85
10090-100
2010
Zobrazit abstrakt
Human respiratory syncytial virus (RSV), a major cause of severe respiratory diseases, efficiently suppresses cellular innate immunity, represented by type I interferon (IFN), using its two unique nonstructural proteins, NS1 and NS2. In a search for their mechanism, NS1 was previously shown to decrease levels of TRAF3 and IKKε, whereas NS2 interacted with RIG-I and decreased TRAF3 and STAT2. Here, we report on the interaction, cellular localization, and functional domains of these two proteins. We show that recombinant NS1 and NS2, expressed in lung epithelial A549 cells, can form homo- as well as heteromers. Interestingly, when expressed alone, substantial amounts of NS1 and NS2 localized to the nuclei and to the mitochondria, respectively. However, when coexpressed with NS2, as in RSV infection, NS1 could be detected in the mitochondria as well, suggesting that the NS1-NS2 heteromer localizes to the mitochondria. The C-terminal tetrapeptide sequence, DLNP, common to both NS1 and NS2, was required for some functions, but not all, whereas only the NS1 N-terminal region was important for IKKε reduction. Finally, NS1 and NS2 both interacted specifically with host microtubule-associated protein 1B (MAP1B). The contribution of MAP1B in NS1 function was not tested, but in NS2 it was essential for STAT2 destruction, suggesting a role of the novel DLNP motif in protein-protein interaction and IFN suppression. | 21795342
 |
Respiratory syncytial virus nonstructural proteins decrease levels of multiple members of the cellular interferon pathways.
Samer Swedan,Alla Musiyenko,Sailen Barik
Journal of virology
83
2009
Zobrazit abstrakt
Viruses of the Paramyxoviridae family, such as the respiratory syncytial virus (RSV), suppress cellular innate immunity represented by type I interferon (IFN) for optimal growth in their hosts. The two unique nonstructural (NS) proteins, NS1 and NS2, of RSV suppress IFN synthesis, as well as IFN function, but their exact targets are still uncharacterized. Here, we investigate if either or both of the NS proteins affect the steady-state levels of key members of the IFN pathway. We found that both NS1 and NS2 decreased the levels of TRAF3, a strategic integrator of multiple IFN-inducing signals, although NS1 was more efficient. Only NS1 reduced IKKepsilon, a key protein kinase that specifically phosphorylates and activates IFN regulatory factor 3. Loss of the TRAF3 and IKKepsilon proteins appeared to involve a nonproteasomal mechanism. Interestingly, NS2 modestly increased IKKepsilon levels. In the IFN response pathway, NS2 decreased the levels of STAT2, the essential transcription factor for IFN-inducible antiviral genes. Preliminary mapping revealed that the C-terminal 10 residues of NS1 were essential for reducing IKKepsilon levels and the C-terminal 10 residues of NS2 were essential for increasing and reducing IKKepsilon and STAT2, respectively. In contrast, deletion of up to 20 residues of the C termini of NS1 and NS2 did not diminish their TRAF3-reducing activity. Coimmunoprecipitation studies revealed that NS1 and NS2 form a heterodimer. Clearly, the NS proteins of RSV, working individually and together, regulate key signaling molecules of both the IFN activation and response pathways. Celý text článku | 19625398
 |