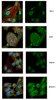
Neural pathways associated with loss of consciousness caused by intracerebral microinjection of GABA A-active anesthetics.
I Sukhotinsky, V Zalkind, J Lu, D A Hopkins, C B Saper, M Devor, I Sukhotinsky, V Zalkind, J Lu, D A Hopkins, C B Saper, M Devor
The European journal of neuroscience
25
1417-36
2007
Zobrazit abstrakt
Anesthesia, slow-wave sleep, syncope, concussion and reversible coma are behavioral states characterized by loss of consciousness, slow-wave cortical electroencephalogram, and motor and sensory suppression. We identified a focal area in the rat brainstem, the mesopontine tegmental anesthesia area (MPTA), at which microinjection of pentobarbital and other GABA(A) receptor (GABA(A)-R) agonists reversibly induced an anesthesia-like state. This effect was attenuated by local pre-treatment with the GABA(A)-R antagonist bicuculline. Using neuroanatomical tracing we identified four pathways ascending from the MPTA that are positioned to mediate electroencephalographic synchronization and loss of consciousness: (i) projections to the intralaminar thalamic nuclei that, in turn, project to the cortex; (ii) projections to several pontomesencephalic, diencephalic and basal forebrain nuclei that project cortically and are considered parts of an ascending arousal system; (iii) a projection to other parts of the subcortical forebrain, including the septal area, hypothalamus, zona incerta and striato-pallidal system, that may indirectly affect cortical arousal and hippocampal theta rhythm; and (iv) modest projections directly to the frontal cortex. Several of these areas have prominent reciprocal projections back to the MPTA, notably the zona incerta, lateral hypothalamus and frontal cortex. We hypothesize that barbiturate anesthetics and related agents microinjected into the MPTA enhance the inhibitory response of local GABA(A)-R-bearing neurons to endogenous GABA released at baseline during wakefulness. This modulates activity in one or more of the identified ascending neural pathways, ultimately leading to loss of consciousness. | 17425568
 |
Hypoglycemia activates arousal-related neurons and increases wake time in adult rats.
Nancy C Tkacs,Yanhua Pan,Gagan Sawhney,Graziella L Mann,Adrian R Morrison
Physiology & behavior
91
2007
Zobrazit abstrakt
Hypoglycemia resulting from excess of exogenous or endogenous insulin elicits central nervous system activation that contributes to counterregulatory hormone secretion. In adult humans without diabetes, hypoglycemia occurring during sleep usually produces cortical activation with awakening. However, in adult humans with type 1 diabetes, hypoglycemic arousal appears blunted or absent. We hypothesized that insulin injection sufficient to produce hypoglycemia would induce awakening in adult male rats. Polysomnographic studies were carried out to characterize the effect of insulin injection on measures of sleep and waking during a circadian time of increased sleep. Compared to a baseline day, insulin treatment more than doubled the time spent awake, from 18.4+/-2.6% after saline injection to 48.0+/-5.5% after insulin. Insulin injection also reduced rapid eye movement sleep (REMS) from 27.3+/-1.8% to 5.6+/-1.3%. The percent of time in non-REM sleep (NREMS) sleep was not different between saline and insulin days, however, NREMS after insulin was fragmented, with increased number and decreased duration of episodes. These electrophysiological data indicate that insulin-induced hypoglycemia is an arousing stimulus in rats, as in nondiabetic adult humans. We also studied the effect of insulin on activation of selected arousal-related neurons using immunohistochemical detection of Fos. Fos-immunoreactivity increased in orexin (OX) neurons after insulin, from 8.7+/-4.9% after saline injection to 37+/-9% after insulin. Basal forebrain cholinergic nuclei also showed increased Fos-immunoreactivity after insulin. These correlated behavioral and histological data provide targets for future studies of the neural pathways underlying hypoglycemic arousal. Celý text článku | 17434543
 |
Platelet-activating factor in the enteric nervous system of the guinea pig small intestine.
Wang, GD; Wang, XY; Hu, HZ; Fang, XC; Liu, S; Gao, N; Xia, Y
American journal of physiology. Gastrointestinal and liver physiology
291
G928-37
2005
Zobrazit abstrakt
Platelet-activating factor (PAF) is a proinflammatory mediator that may influence neuronal activity in the enteric nervous system (ENS). Electrophysiology, immunofluorescence, Western blot analysis, and RT-PCR were used to study the action of PAF and the expression of PAF receptor (PAFR) in the ENS. PAFR immunoreactivity (IR) was expressed by 6.9% of the neurons in the myenteric plexus and 14.5% of the neurons in the submucosal plexus in all segments of the guinea pig intestinal tract as determined by double staining with anti-human neuronal protein antibody. PAFR IR was found in 6.1% of the neurons with IR for calbindin, 35.8% of the neurons with IR for neuropeptide Y (NPY), 30.6% of the neurons with IR for choline acetyltransferase (ChAT), and 1.96% of the neurons with IR for vasoactive intestinal peptide (VIP) in the submucosal plexus. PAFR IR was also found in 1.5% of the neurons with IR for calbindin, 51.1% of the neurons with IR for NPY, and 32.9% of the neurons with IR for ChAT in the myenteric plexus. In the submucosal plexus, exposure to PAF (200-600 nM) evoked depolarizing responses (8.2 +/- 3.8 mV) in 12.4% of the neurons with S-type electrophysiological behavior and uniaxonal morphology and in 12.5% of the neurons with AH-type electrophysiological behavior and Dogiel II morphology, whereas in the myenteric preparations, depolarizing responses were elicited by a similar concentration of PAF in 9.5% of the neurons with S-type electrophysiological behavior and uniaxonal morphology and in 12.0% of the neurons with AH-type electrophysiological behavior and Dogiel II morphology. The results suggest that subgroups of secreto- and musculomotor neurons in the submucosal and myenteric plexuses express PAFR. Coexpression of PAFR IR with ChAT IR in the myenteric plexus and ChAT IR and VIP IR in the submucosal plexus suggests that PAF, after release in the inflamed bowel, might act to elevate the excitability of submucosal secretomotor and myenteric musculomotor neurons. Enhanced excitability of motor neurons might lead to a state of neurogenic secretory diarrhea. | 17030900
 |
Viral-induced spinal motor neuron death is non-cell-autonomous and involves glutamate excitotoxicity.
Darman, J; Backovic, S; Dike, S; Maragakis, NJ; Krishnan, C; Rothstein, JD; Irani, DN; Kerr, DA
The Journal of neuroscience : the official journal of the Society for Neuroscience
24
7566-75
2004
Zobrazit abstrakt
Neuroadapted Sindbis virus (NSV) is a neurotropic virus capable of inducing the death of spinal motor neurons in mice and rats. In this study we investigated the mechanisms that underlie NSV-induced motor neuron death. We found that many degenerating spinal motor neurons were not infected directly with NSV, suggesting that bystander cell death occurs. An excitotoxic mechanism was confirmed when blockade of calcium-permeable AMPA receptors attenuated motor neuron death both in vitro and in vivo. Blockade of astroglial glutamate reuptake potentiated NSV-induced motor neuron loss in vivo, suggesting that astrocyte-mediated removal of perisynaptic glutamate is important in limiting NSV-induced excitotoxic injury. Astroglial glutamate transport was reduced markedly in the spinal cord during NSV infection, in advance of motor neuron injury in susceptible mice. In contrast, we found 5.6-fold elevated glutamate uptake in the spinal cords of mice resistant to NSV-induced paralysis. Likewise, minocycline markedly increased spinal cord glutamate transport and protected mice from NSV-induced motor neuron death. These studies suggest that NSV infection triggers a cascade of events in the spinal cord resulting in impaired astrocytic glutamate transport and excitotoxic injury of motor neurons mediated via calcium-permeable AMPA receptors. Similar changes may occur in other motor neuron disorders such as amyotrophic lateral sclerosis or West Nile Virus-induced poliomyelitis, suggesting a common tissue injury pathway. | 15329404
 |
Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury.
Douglas A Kerr, Jerònia Lladó, Michael J Shamblott, Nicholas J Maragakis, David N Irani, Thomas O Crawford, Chitra Krishnan, Sonny Dike, John D Gearhart, Jeffrey D Rothstein
The Journal of neuroscience : the official journal of the Society for Neuroscience
23
5131-40
2003
Zobrazit abstrakt
We have investigated the potential of human pluripotent cells to restore function in rats paralyzed with a virus-induced motor neuronopathy. Cells derived from embryonic germ cells, termed embryoid body-derived (EBD) cells, introduced into the CSF were distributed extensively over the rostrocaudal length of the spinal cord and migrated into the spinal cord parenchyma in paralyzed, but not uninjured, animals. Some of the transplanted human cells expressed the neuroglial progenitor marker nestin, whereas others expressed immunohistochemical markers characteristic of astrocytes or mature neurons. Rare transplanted cells developed immunoreactivity to choline acetyltransferase (ChAT) and sent axons into the sciatic nerve as detected by retrograde labeling. Paralyzed animals transplanted with EBD cells partially recovered motor function 12 and 24 weeks after transplantation, whereas control animals remained paralyzed. Semi-quantitative analysis revealed that the efficiency of neuronal differentiation and extension of neurites could not account for the functional recovery. Rather, transplanted EBD cells protected host neurons from death and facilitated reafferentation of motor neuron cell bodies. In vitro, EBD cells secrete transforming growth factor-alpha (TGF-alpha) and brain-derived neurotrophic factor (BDNF). Neutralizing antibodies to TGF-alpha and to BDNF abrogated the ability of EBD-conditioned media to sustain motor neuron survival in culture, whereas neutralizing antibodies to BDNF eliminated the axonal outgrowth from spinal organotypics observed with direct coculture of EBD cells. We conclude that cells derived from human pluripotent stem cells have the capacity to restore neurologic function in animals with diffuse motor neuron disease via enhancement of host neuron survival and function. | 12832537
 |