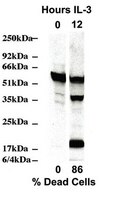
BH3-only protein BIM mediates heat shock-induced apoptosis.
Mahajan, IM; Chen, MD; Muro, I; Robertson, JD; Wright, CW; Bratton, SB
PloS one
9
e84388
2014
Zobrazit abstrakt
Acute heat shock can induce apoptosis through a canonical pathway involving the upstream activation of caspase-2, followed by BID cleavage and stimulation of the intrinsic pathway. Herein, we report that the BH3-only protein BIM, rather than BID, is essential to heat shock-induced cell death. We observed that BIM-deficient cells were highly resistant to heat shock, exhibiting short and long-term survival equivalent to Bax(-/-)Bak(-/-) cells and better than either Bid(-/-) or dominant-negative caspase-9-expressing cells. Only Bim(-/-) and Bax(-/-)Bak(-/-) cells exhibited resistance to mitochondrial outer membrane permeabilization and loss of mitochondrial inner membrane potential. Moreover, while dimerized caspase-2 failed to induce apoptosis in Bid(-/-) cells, it readily did so in Bim(-/-) cells, implying that caspase-2 kills exclusively through BID, not BIM. Finally, BIM reportedly associates with MCL-1 following heat shock, and Mcl-1(-/-) cells were indeed sensitized to heat shock-induced apoptosis. However, pharmacological inhibition of BCL-2 and BCL-X(L) with ABT-737 also sensitized cells to heat shock, most likely through liberation of BIM. Thus, BIM mediates heat shock-induced apoptosis through a BAX/BAK-dependent pathway that is antagonized by antiapoptotic BCL-2 family members. | | 24427286
 |
An L-tyrosine derivative and PPARgamma agonist, GW7845, activates a multifaceted caspase cascade in bone marrow B cells.
Schlezinger, JJ; Emberley, JK; Bissonnette, SL; Sherr, DH
Toxicological sciences : an official journal of the Society of Toxicology
98
125-36
2007
Zobrazit abstrakt
Apoptosis is a critical event in the deletion of B lymphocytes prior to their migration to the periphery. Synthetic peroxisome proliferator activated receptor gamma (PPARgamma) agonists, including the drug GW7845 and the environmental contaminant mono-(2-ethylhexyl) phthalate, as well as an endogenous ligand, 15-deoxy-Delta(12,14)-prostaglandin J(2), induce clonally unrestricted apoptosis in pro/pre-B cells. Considering that PPARgamma agonists are used clinically for the treatment of diabetes and postulated to be useful as chemotherapeutics, we used GW7845 as a model PPARgamma agonist to examine the mechanism of cell death that may contribute to tumor killing as well as normal bone marrow B lymphocyte toxicity. GW7845 induced rapid mitochondrial membrane depolarization and release of cytochrome c, along with nearly concurrent activation of capases-2, -3, -8, and -9 in primary pro-B cells and BU-11 cells, a nontransformed pro/pre-B cell line. GW7845-induced apoptosis was reduced significantly in Bax-deficient and Apaf-1 mutant primary pro-B cells, supporting the conclusion that GW7845-induced apoptosis is mitochondria- and apoptosome-dependent. Using benzyloxycarbonyl-VAD-fluoromethyl ketone (VAD-FMK) as a pan-caspase inhibitor, we demonstrated that an initial cytochrome c release occurred independently of caspase activation and that only caspase-9 activation was partially caspase independent. The attenuation of GW7845-induced apoptosis by multiple FMK-labeled peptide sequences suggests that multiple caspase pathways are responsible for initiating and executing apoptosis. The strong activation of Bid provides a mechanism by which caspases-2, -3, and -8 may amplify the apoptotic signal. These data support the hypothesis that pharmacologic concentrations of PPARgamma agonists induce an intrinsic apoptotic pathway that is driven in normal bone marrow B cells by multiple amplification loops. | | 17400580
 |
Specific caspase interactions and amplification are involved in selective neuronal vulnerability in Huntington's disease.
Hermel, E; Gafni, J; Propp, SS; Leavitt, BR; Wellington, CL; Young, JE; Hackam, AS; Logvinova, AV; Peel, AL; Chen, SF; Hook, V; Singaraja, R; Krajewski, S; Goldsmith, PC; Ellerby, HM; Hayden, MR; Bredesen, DE; Ellerby, LM
Cell death and differentiation
11
424-38
2004
Zobrazit abstrakt
Huntington's disease (HD) is an autosomal dominant progressive neurodegenerative disorder resulting in selective neuronal loss and dysfunction in the striatum and cortex. The molecular pathways leading to the selectivity of neuronal cell death in HD are poorly understood. Proteolytic processing of full-length mutant huntingtin (Htt) and subsequent events may play an important role in the selective neuronal cell death found in this disease. Despite the identification of Htt as a substrate for caspases, it is not known which caspase(s) cleaves Htt in vivo or whether regional expression of caspases contribute to selective neuronal cells loss. Here, we evaluate whether specific caspases are involved in cell death induced by mutant Htt and if this correlates with our recent finding that Htt is cleaved in vivo at the caspase consensus site 552. We find that caspase-2 cleaves Htt selectively at amino acid 552. Further, Htt recruits caspase-2 into an apoptosome-like complex. Binding of caspase-2 to Htt is polyglutamine repeat-length dependent, and therefore may serve as a critical initiation step in HD cell death. This hypothesis is supported by the requirement of caspase-2 for the death of mouse primary striatal cells derived from HD transgenic mice expressing full-length Htt (YAC72). Expression of catalytically inactive (dominant-negative) forms of caspase-2, caspase-7, and to some extent caspase-6, reduced the cell death of YAC72 primary striatal cells, while the catalytically inactive forms of caspase-3, -8, and -9 did not. Histological analysis of post-mortem human brain tissue and YAC72 mice revealed activation of caspases and enhanced caspase-2 immunoreactivity in medium spiny neurons of the striatum and the cortical projection neurons when compared to controls. Further, upregulation of caspase-2 correlates directly with decreased levels of brain-derived neurotrophic factor in the cortex and striatum of 3-month YAC72 transgenic mice and therefore suggests that these changes are early events in HD pathogenesis. These data support the involvement of caspase-2 in the selective neuronal cell death associated with HD in the striatum and cortex. | Western Blotting | 14713958
 |
Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis.
Mancini, M, et al.
J. Cell Biol., 149: 603-12 (2000)
1999
Zobrazit abstrakt
Caspases are an extended family of cysteine proteases that play critical roles in apoptosis. Animals deficient in caspases-2 or -3, which share very similar tetrapeptide cleavage specificities, exhibit very different phenotypes, suggesting that the unique features of individual caspases may account for distinct regulation and specialized functions. Recent studies demonstrate that unique apoptotic stimuli are transduced by distinct proteolytic pathways, with multiple components of the proteolytic machinery clustering at distinct subcellular sites. We demonstrate here that, in addition to its nuclear distribution, caspase-2 is localized to the Golgi complex, where it cleaves golgin-160 at a unique site not susceptible to cleavage by other caspases with very similar tetrapeptide specificities. Early cleavage at this site precedes cleavage at distal sites by other caspases. Prevention of cleavage at the unique caspase-2 site delays disintegration of the Golgi complex after delivery of a pro-apoptotic signal. We propose that the Golgi complex, like mitochondria, senses and integrates unique local conditions, and transduces pro-apoptotic signals through local caspases, which regulate local effectors. | | 10791974
 |
Prodomain-dependent nuclear localization of the caspase-2 (Nedd2) precursor. A novel function for a caspase prodomain.
Colussi, P A, et al.
J. Biol. Chem., 273: 24535-42 (1998)
1998
Zobrazit abstrakt
Caspases are cysteine proteases that play an essential role in apoptosis by cleaving several key cellular proteins. Despite their function in apoptosis, little is known about where in the cell they are localized and whether they are translocated to specific cellular compartments upon activation. In the present paper, using Aequorea victoria green fluorescent protein fusion constructs, we have determined the localization of Nedd2 (mouse caspase-2) and show that both precursor and processed caspase-2 localize to the cytoplasmic and the nuclear compartments. We demonstrate that the nuclear localization of caspase-2 is strictly dependent on the presence of the prodomain. A caspase-2 prodomain-green fluorescent protein localized to dot- and fiber-like structures mostly in the nucleus, whereas a protein lacking the prodomain was largely concentrated in the cytoplasm. We also show that an amino-terminal fusion of the prodomain of caspase-2 to caspase-3 mediates nuclear transport of caspase-3, which is normally localized in the cytoplasm. These results suggest that, in addition to roles in dimerization and recruitment through adaptors, the caspase-2 prodomain has a novel function in nuclear transport. | | 9733748
 |