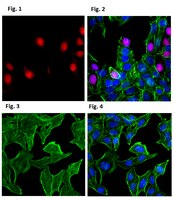
Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation.
Tsuji, Takanori, et al.
Cancer Res., 65: 1352-60 (2005)
2004
Zobrazit abstrakt
Angiogenin is an angiogenic protein known to play a role in rRNA transcription in endothelial cells. Nuclear translocation of angiogenin in endothelial cells decreases as cell density increases and ceases when cells are confluent. Here we report that angiogenin is constantly translocated to the nucleus of HeLa cells in a cell density-independent manner. Down-regulation of angiogenin expression by antisense and RNA interference results in a decrease in rRNA transcription, ribosome biogenesis, proliferation, and tumorigenesis both in vitro and in vivo. Exogenous angiogenin rescues the cells from antisense and RNA interference inhibition. The results showed that angiogenin is constitutively translocated into the nucleus of HeLa cells where it stimulates rRNA transcription. Thus, besides its angiogenic activity, angiogenin also plays a role in cancer cell proliferation. | 15735021
 |
Chimeric anti-angiogenin antibody cAb 26-2F inhibits the formation of human breast cancer xenografts in athymic mice.
Piccoli, R, et al.
Proc. Natl. Acad. Sci. U.S.A., 95: 4579-83 (1998)
1998
Zobrazit abstrakt
Angiogenin (Ang), an inducer of neovascularization, is secreted by several types of human tumor cells and appears critical for their growth. The murine anti-Ang monoclonal antibody (mAb) 26-2F neutralizes the activities of Ang and dramatically prevents the establishment and metastatic dissemination of human tumor cell xenografts in athymic mice. However, for use clinically, the well-documented problem of the human anti-globulin antibody response known to occur with murine antibodies requires resolution. As a result, chimeric as well as totally humanized antibodies are currently being evaluated as therapeutic agents for the treatment of several pathological conditions, including malignancy. Therefore, we have constructed a chimeric mouse/human antibody based on the structure of mAb 26-2F. Complementary DNAs from the light and heavy chain variable regions of mAb 26-2F were cloned, sequenced, and genetically engineered by PCR for subcloning into expression vectors that contain human constant region sequences. Transfection of these vectors into nonproducing mouse myeloma cells resulted in the secretion of fully assembled tetrameric molecules. The chimeric antibody (cAb 26-2F) binds to Ang and inhibits its ribonucleolytic and angiogenic activities as potently as mAb 26-2F. Furthermore, the capacities of cAb 26-2F and its murine counterpart to suppress the formation of human breast cancer tumors in athymic mice are indistinguishable. Thus cAb 26-2F, with its retained neutralization capability and likely decreased immunogenicity, may be of use clinically for the treatment of human cancer and related disorders where pathological angiogenesis is a component. | 9539780
 |
A monoclonal antibody to human angiogenin. Inhibition of ribonucleolytic and angiogenic activities and localization of the antigenic epitope.
Fett, J W, et al.
Biochemistry, 33: 5421-7 (1994)
1993
Zobrazit abstrakt
A monoclonal antibody (mAb) to human angiogenin, a protein that induces formation of new blood vessels, was produced by somatic cell fusion techniques and designated as 26-2F. It is an IgGl kappa whose binding affinity, expressed as an IC50, is (1.6 +/- 0.1) x 10(-9) M as determined by a competition radioimmunoassay. mAb 26-2F neutralizes the ribonucleolytic activity of angiogenin as assessed by in vitro protein synthesis and tRNA degradation assays. It also effectively inhibits neovascularization induced by angiogenin on the chick chorioallantoic membrane. Epitope mapping indicates that the binding region of angiogenin recognized by mAb 26-2F is discontinuous and involves both Trp-89 and residues in the segment 38-41. This epitope is formed by two surface loops which are juxtaposed in the three-dimensional structure of human angiogenin recently determined by X-ray crystallography. Thus mAb 26-2F, along with similar antibodies under investigation, will facilitate structure/function studies of angiogenin, help define its physiological role, and lead to an understanding of the consequences of its inhibition in pathological situations in which angiogenin may be involved. | 7514035
 |