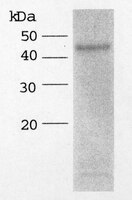
The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage.
Tortorella, M, et al.
J. Biol. Chem., 275: 25791-7 (2000)
1999
Zobrazit abstrakt
Aggrecanase-1 (ADAMTS-4) is a member of the a disintegrin and metalloprotease with thrombospondin motifs (ADAMTS) protein family that was recently identified. Aggrecanase-1 is one of two ADAMTS cartilage-degrading enzymes purified from interleukin-1-stimulated bovine nasal cartilage (Tortorella, M. D., Burn, T. C., Pratta, M. A. , Abbaszade, I., Hollis, J. M., Liu, R., Rosenfeld, S. A., Copeland, R. A., Decicco, C. P., Wynn, R., Rockwell, A., Yang, F., Duke, J. L., Solomon, K., George, H., Bruckner, R., Nagase, H., Itoh, Y., Ellis, D. M., Ross, H., Wiswall, B. H., Murphy, K., Hillman, M. C., Jr., Hollis, G. F., and Arner, E.C. (1999) Science 284, 1664-1666; 2 Abbaszade, I., Liu, R. Q., Yang, F., Rosenfeld, S. A., Ross, O. H., Link, J. R., Ellis, D. M., Tortorella, M. D., Pratta, M. A., Hollis, J. M., Wynn, R., Duke, J. L., George, H. J., Hillman, M. C., Jr., Murphy, K., Wiswall, B. H., Copeland, R. A., Decicco, C. P., Bruckner, R., Nagase, H., Itoh, Y., Newton, R. C., Magolda, R. L., Trzaskos, J. M., and Burn, T. C. (1999) J. Biol. Chem. 274, 23443-23450). The aggrecan products generated by this enzyme are found in cartilage cultures stimulated with cytokines and in synovial fluid from patients with arthritis, suggesting that aggrecanase-1 may be important in diseases involving cartilage destruction. Here we demonstrate that the thrombospondin type-1 (TSP-1) motif located within the C terminus of aggrecanase-1 binds to the glycosaminoglycans of aggrecan. Data from several studies indicate that this binding of aggrecanase-1 to aggrecan through the TSP-1 motif is necessary for enzymatic cleavage of aggrecan. 1) A truncated form of aggrecanase-1 lacking the TSP-1 motif was not effective in cleaving aggrecan. 2) Several peptides representing different regions of the TSP-1 motif effectively blocked aggrecanase-1 cleavage of aggrecan by preventing the enzyme from binding to the substrate. 3) Aggrecanase-1 was not effective in cleaving glycosaminoglycan-free aggrecan. Taken together, these data suggest that the TSP-1 motif of aggrecanase-1 is critical for substrate recognition and cleavage. | 10827174
 |