196878 Sigma-Aldrich(±)-Bay K 8644 - CAS 93468-89-4 - Calbiochem
Synthetic dihydropyridine derivative that acts as an active Ca2+ slow channel agonist in neuroendocrine, muscle, thyroid and other cell types.
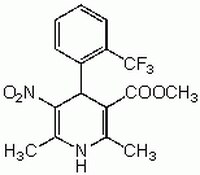
More>> Synthetic dihydropyridine derivative that acts as an active Ca2+ slow channel agonist in neuroendocrine, muscle, thyroid and other cell types. Less<<Synonyms: 1,4-Dihydro-2,6-dimethyl-5-nitro-4-[2ʹ-(trifluoromethyl)phenyl]-3-pyridinecarboxylic Acid Methyl Ester
Recommended Products
Přehled
| Replacement Information |
|---|
Tabulka spec. kláve
| CAS # | Empirical Formula |
|---|---|
| 93468-89-4 | C₁₆H₁₅F₃N₂O₄ |
Products
| Katalogové číslo | Balení | ks/bal. | |
|---|---|---|---|
| 196878-1MG | Plastová ampulka | 1 mg | |
| 196878-5MG | Plastová ampulka | 5 mg |
| Product Information | |
|---|---|
| CAS number | 93468-89-4 |
| ATP Competitive | N |
| Form | Yellow solid |
| Hill Formula | C₁₆H₁₅F₃N₂O₄ |
| Chemical formula | C₁₆H₁₅F₃N₂O₄ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | L-type Ca2+ channel |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalogové číslo | GTIN |
| 196878-1MG | 04055977206609 |
| 196878-5MG | 04055977206616 |
Documentation
(±)-Bay K 8644 - CAS 93468-89-4 - Calbiochem MSDS
| Title |
|---|
(±)-Bay K 8644 - CAS 93468-89-4 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 196878 |
References
| Přehled odkazů |
|---|
| Wang, W., et al. 2009. Proc. Natl. Acad. Sci. USA 106, 1427. Weigl, L.G., et al. 2000. J. Physiol. 525 (pt. 2), 461. Vannier, C., et al. 1995. Am. J. Physiol. 268, L201. Bechem, M., and Hoffmann, H. 1993. Pflugers Arch. 424, 343. Triggle, D.J., and Rompe, D. 1989. Trends Pharmacol. Sci. 10, 507. Takasu, N., et al. 1987. Biochem. Biophys. Res. Commun. 143, 1107. Tagliatela, M., et al. 1986. Brain Res. 381, 356. Franckowiak, G., et al. 1985. Eur. J. Pharmacol. 114, 223. |