426100 Sigma-AldrichLactacystin, Synthetic - CAS 1258004-00-0 - Calbiochem
Lactacystin, Synthetic, CAS 1258004-00-0, is an irreversible inhibitor of 20S proteasome inhibitor (IC₅₀ = 500 nM). A covalent inhibitor of the chymotrypsin & trypsin-like activities of proteasome.
More>> Lactacystin, Synthetic, CAS 1258004-00-0, is an irreversible inhibitor of 20S proteasome inhibitor (IC₅₀ = 500 nM). A covalent inhibitor of the chymotrypsin & trypsin-like activities of proteasome. Less<<Synonyms: Proteasome Inhibitor VI
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
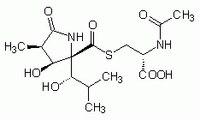
| 1258004-00-0 | C₁₅H₂₄N₂O₇S |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 426100-200UG |
|
Plastic ampoule | 200 μg |
|
— |
| Product Information | |
|---|---|
| CAS number | 1258004-00-0 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₁₅H₂₄N₂O₇S |
| Chemical formula | C₁₅H₂₄N₂O₇S |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 426100-200UG | 04055977187328 |
Documentation
Lactacystin, Synthetic - CAS 1258004-00-0 - Calbiochem SDS
| Title |
|---|
Lactacystin, Synthetic - CAS 1258004-00-0 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 426100 |
References
| Reference overview |
|---|
| Keyomarsi, K., et al. 2011. Cell. Biol. Toxicol. 27, 123. Adams, J., and Stein, R. 1996. Ann. Rep. Med. Chem. 31, 279. Dick, L.R., et al. 1996. J. Biol. Chem. 271, 7273. Oda, K., et al. 1996. Biochem. Biophys. Res. Commun. 219, 800. Fenteany, G., et al. 1995. Science 268, 726. Imajoh-Ohmi, S., et al. 1995. Biochem. Biophys. Res. Commun. 217, 1070. Jensen, T.J., et al. 1995. Cell 83, 129. Katagiri, M.M., et al. 1995. J. Antibiot. 48, 344. Mori, S., et al. 1995. J. Biol. Chem. 270, 29447. Tanaka, H., et al. 1995. Biochem. Biophys. Res. Commun. 216, 291. Fenteany, G., et al. 1994. Proc. Natl. Acad. Sci. USA 91, 3358. Omura, S., et al. 1991. J. Antibiot. 44, 117. |
Brochure
| Title |
|---|
| Caspases and other Apoptosis Related Tools Brochure |
| Proteasomes Technical Bulletin |
Citations
| Title | |
|---|---|
|
|
| Data Sheet | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|