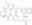178276 Sigma-AldrichApicidin, Fusarium sp. - Calbiochem
A potent, cell-permeable inhibitor of histone deacetylase (IC₅₀= 700 pM for parasitic histone deactetylase) that also exibits antiprotozoal and potential anti-malarial properties.
More>> A potent, cell-permeable inhibitor of histone deacetylase (IC₅₀= 700 pM for parasitic histone deactetylase) that also exibits antiprotozoal and potential anti-malarial properties. Less<<Synonyms: cyclo-[L-(2-Amino-8-oxodecanoyl)-L-(N-methoxytryptophan)-L-isoleucyl-D-pipecolinyl], HDAC Inhibitor XI
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₃₄H₄₉N₅O₆ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 178276-5MG |
|
Alu drum | 5 mg |
|
— |
| Product Information | |
|---|---|
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₃₄H₄₉N₅O₆ |
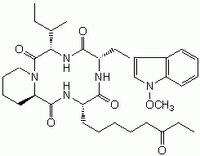
| Chemical formula | C₃₄H₄₉N₅O₆ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Histone deacetylase |
| Primary Target IC<sub>50</sub> | 700 pM for parasitic histone deactetylase |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 178276-5MG | 04055977222418 |
Documentation
Apicidin, Fusarium sp. - Calbiochem SDS
| Title |
|---|
Apicidin, Fusarium sp. - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 178276 |
References
| Reference overview |
|---|
| Colletti, S.L., et al. 2001. Bioorg. Med. Chem. Lett. 11, 107. Kim, J.S., et al. 2001. Biochem. Biophys. Res. Commun. 281, 866. Andrews, K.T., et al. 2000. Int. J. Parasitol. 30, 761. Kim, M.S., et al. 2000. Cancer Lett. 157, 23. Darkin-Rattray, S.J., et al. 1996. Proc. Natl. Acad. Sci. USA 93, 13143. |
Technical Info
| Title |
|---|
| White Paper - The Message in the Marks: Deciphering Cancer Epigenetics |