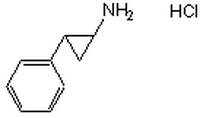
616431 Sigma-AldrichTranylcypromine, HCl - CAS 1986-47-6 - Calbiochem
A cell-permeable phenylcyclopropylamine that inhibits the monoamine oxidase and histone demethylase activities.
More>> A cell-permeable phenylcyclopropylamine that inhibits the monoamine oxidase and histone demethylase activities. Less<<同义词: Histone Lysine Demethylase Inhibitor I, LSD Inhibitor V, Monoamine Oxidase Inhibitor I, Parnate, 2-PCPA, TCP, (1R)-2-Phenylcyclopropan-1-amine, HCl, trans-2-Phenylcyclopropylamine, HCl, BHC110 Inhibitor V, LSD2 Inhibitor I, MOA Inhibitor I, KDM1 Inhibitor V, LSD1 Inhibitor V
Recommended Products
概述
| Replacement Information |
|---|
Products
| 产品目录编号 | 包装 | 数量 / 包装 | |
|---|---|---|---|
| 616431-500MGCN | 玻璃瓶 | 500 mg |
| Description | |
|---|---|
| Overview | A cell-permeable phenylcyclopropylamine that inhibits the monoamine oxidase and histone demethylase activities, respectively, of MAO A/B (Ki = 101.9 and 16.0 µM, respectively) and LSD1/2 (Ki = 242.7 and 180.0 µM, respectively), four members of a flavin-dependent amine oxidase family enzymes, by a covalent adduct formation with the enzyme-bound FAD. In addition to preventing LSD1-CoREST (Corepressor of RE1-Silencing Transcription factor) complex-mediated H3K4 demethylation (IC50 < 2 µM), TCP also inhibits LSD1-HCF-1 (Host Cell Factor-1) complex-mediated H3K9 demethylase activity, which is demonstrated to be an essential mechanism for the replication and latent infection of the α-herpesviruses HSV and VZV. The combined treatment of 2 µM TCP and 10 µM CHIR99061 (Cat. No. 361559) is reported to enable the reprogramming of Oct4/Klf4-transduced primary HNEKs (Human Neonatal Epidermal Keratinocytes) into iPS (induced Pluripotent Stem) cells, albeit at a 100-time lower efficiency as seen in cultures transduced with 4-TFs (Oct44, Klf4, Sox2, and c-Myc). |
| Catalogue Number | 616431 |
| Brand Family | Calbiochem® |
| Synonyms | Histone Lysine Demethylase Inhibitor I, LSD Inhibitor V, Monoamine Oxidase Inhibitor I, Parnate, 2-PCPA, TCP, (1R)-2-Phenylcyclopropan-1-amine, HCl, trans-2-Phenylcyclopropylamine, HCl, BHC110 Inhibitor V, LSD2 Inhibitor I, MOA Inhibitor I, KDM1 Inhibitor V, LSD1 Inhibitor V |
| Product Information | |
|---|---|
| CAS number | 1986-47-6 |
| Form | White to yellow solid |
| Hill Formula | C₉H₁₁N •HCl |
| Chemical formula | C₉H₁₁N •HCl |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥97% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 产品目录编号 | GTIN |
| 616431-500MGCN | 04055977185768 |
Documentation
Tranylcypromine, HCl - CAS 1986-47-6 - Calbiochem MSDS
| 职位 |
|---|
Tranylcypromine, HCl - CAS 1986-47-6 - Calbiochem 分析证书
| 标题 | 批号 |
|---|---|
| 616431 |
参考
| 参考信息概述 |
|---|
| Liang, Y., et al. 2009. Nat. Med. 15, 1312. Li, W., et al. 2009. Stem Cells 27, 2992. Karytinos, A., et al. 2009. J. Biol. Chem. 284, 17775. Hruschka, S., et al. 2008. Bioorg. Med. Chem. 16, 7148. Schmidt, D.M., and McCafferty, D.G. 2007. Biochemistry 46, 4408. Lee, M.G., et al. 2006. Chem. Biol. 13, 563. Yoshida, S., et al. 2004. Bioorg. Med. Chem. 12, 2645. |
技术信息
| 标题 |
|---|
| White Paper - The Message in the Marks: Deciphering Cancer Epigenetics |
| 数据表 | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|