574102 Sigma-AldrichSulindac Sulfide - CAS 32004-67-4 - Calbiochem
A cell-permeable, active metabolite of Sulindac.
More>> A cell-permeable, active metabolite of Sulindac. Less<<MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents.
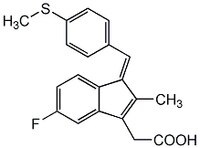
同义词: (Z)-5-Fluoro-2-methyl-1-[p-(methylthio)benzylidene]indene-3-acetic Acid
Recommended Products
概述
| Replacement Information |
|---|
重要规格表
| CAS # | Empirical Formula |
|---|---|
| 32004-67-4 | C₂₀H₁₇FO₂S |
Products
| 产品目录编号 | 包装 | 数量 / 包装 | |
|---|---|---|---|
| 574102-5MGCN | 塑胶安瓿;塑胶针药瓶 | 5 mg |
| Description | |
|---|---|
| Overview | A cell-permeable, active metabolite of Sulindac (Cat. No. 574100). Strongly inhibits Ras-induced malignant transformation and Ras/Raf-dependent transactivation. Also decreases the Ras-induced activation of c-Raf-1 kinase. Inhibits growth and induces apoptosis in prostate cancer cell lines. Selectively inhibits COX-1 (ID50 = 500 nM) compared to prostaglandin H synthase-2 (PGHS-2; COX-2; ID50 = 14 µM). Preferentially inhibits the secretion of Aβ42 in CHO cells stably transfected with both APP75, and the PS1 mutant M146L. |
| Catalogue Number | 574102 |
| Brand Family | Calbiochem® |
| Synonyms | (Z)-5-Fluoro-2-methyl-1-[p-(methylthio)benzylidene]indene-3-acetic Acid |
| Product Information | |
|---|---|
| CAS number | 32004-67-4 |
| ATP Competitive | N |
| Form | Yellow-orange solid |
| Hill Formula | C₂₀H₁₇FO₂S |
| Chemical formula | C₂₀H₁₇FO₂S |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | COX-1 |
| Primary Target IC<sub>50</sub> | ID50 = 500 nM against COX-1 |
| Purity | ≥98% by TLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Storage and Shipping Information | |
|---|---|
| Ship Code | Ambient Temperature Only |
| Toxicity | Harmful & Carcinogenic / Teratogenic |
| Storage | +15°C to +30°C |
| Do not freeze | Ok to freeze |
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 产品目录编号 | GTIN |
| 574102-5MGCN | 04055977189551 |
Documentation
Sulindac Sulfide - CAS 32004-67-4 - Calbiochem MSDS
| 职位 |
|---|
Sulindac Sulfide - CAS 32004-67-4 - Calbiochem 分析证书
| 标题 | 批号 |
|---|---|
| 574102 |
参考
| 参考信息概述 |
|---|
| Weggen, S., et al. 2001. Nature 414, 212. Lim, J.T., et al. 1999. Biochem. Pharmacol. 58, 1097. Herrmann, C., et al. 1998. Oncogene 17, 1769. Picariello, L., et al. 1998. Eur. J. Pharmacol. 360, 105. Arber, N., et al. 1997. Gastroenterology 113, 1892. Meade, E.A., et al. 1993. J. Biol. Chem. 268, 6610. |