557366 Sigma-AldrichRosiglitazone - CAS 155141-29-0 - Calbiochem
A thiazolidinedione compound that acts as an anti-diabetic agent and serves as a potent and selective agonist of peroxisome proliferator-activated receptor-γ (PPARγ) (Kd ~40 nM) in fat cells.
More>> A thiazolidinedione compound that acts as an anti-diabetic agent and serves as a potent and selective agonist of peroxisome proliferator-activated receptor-γ (PPARγ) (Kd ~40 nM) in fat cells. Less<<Rosiglitazone - CAS 155141-29-0 - Calbiochem MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents.
同义词: 5-[4-(2-[methyl(pyridin-2-yl)amino]ethoxy)benzyl]thiazolidine-2,4-dione, BRL49653, Avandia, AMPK Signaling Activator X, PPAR Agonist X, PPARγ Agonist IX
Recommended Products
概述
| Replacement Information |
|---|
重要规格表
| CAS # | Empirical Formula |
|---|---|
| 155141-29-0 | C₁₈H₁₉N₃O₃S.C₄H₄O₄ |
Products
| 产品目录编号 | 包装 | 数量 / 包装 | |
|---|---|---|---|
| 557366-10MGCN | 玻璃瓶 | 10 mg |
| Product Information | |
|---|---|
| CAS number | 155141-29-0 |
| Form | White powder |
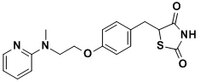
| Hill Formula | C₁₈H₁₉N₃O₃S.C₄H₄O₄ |
| Chemical formula | C₁₈H₁₉N₃O₃S.C₄H₄O₄ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥99% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 产品目录编号 | GTIN |
| 557366-10MGCN | 04055977268027 |
Documentation
Rosiglitazone - CAS 155141-29-0 - Calbiochem MSDS
| 职位 |
|---|
Rosiglitazone - CAS 155141-29-0 - Calbiochem 分析证书
| 标题 | 批号 |
|---|---|
| 557366 |
参考
| 参考信息概述 |
|---|
| Araki, T., et al. 2011. PPAR Res. 2011, 926438. Sozio, M. S., et al. 2011. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G739. Gerstein, H., et al. 2006. Drug 368, 9541. Mohanty, P., 2004. Journ. Clin. Endocrin. & Metab. 89(6), 2728-2735. Fryer, L., et al. 2002. J Biol Chem 277, 25226. Goldberg, R., et al. 1999. Drug. 57, 921. Young, P., et al. 1998. Journ. Pharm. Exp. Ther. 284, 751. Willson, T., et al. 1996. J. Med. Chem.39, 665; Lehmann, J., et al., 1995. JBC 270, 12953. Cantello, B., et al., 1994. Bioorg. Med. Chem. Lett. 4, 1181. |