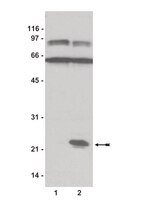
Signaling of hepatocyte growth factor/scatter factor (HGF) to the small GTPase Rap1 via the large docking protein Gab1 and the adapter protein CRKL.
Sakkab, D, et al.
J. Biol. Chem., 275: 10772-8 (2000)
2000
显示摘要
Hepatocyte growth factor (HGF; scatter factor) is a multipotent protein with mitogenic, motogenic, and developmental functions. Upon activation, the HGF-receptor c-Met binds and phosphorylates the multisite docking protein Gab1. Besides binding motifs for phosphatidylinositol 3-kinase and Grb2, Gab 1 contains multiple Tyr-X-X-Pro (YXXP) motifs which, when phosphorylated, are potential binding sites for the adapter proteins c-Crk and Crk-like (CRKL). Stimulation of human embryonic kidney cells (HEK293) with HGF leads to Gab1 association with CRKL. The Gab1-CRKL interaction requires both, the SH2 domain of CRKL and the region containing the YXXP motifs in Gab1. CRKL binds via its first SH3 domain to several downstream signal transducers, including C3G an activator of the small GTPase Rap1. Indeed, Rap1 was rapidly activated after HGF stimulation of HEK293 cells. Rap1 activation through HGF was suppressed through transfection of a truncated C3G protein which only contains the SH3-binding motifs of C3G. Transfection of nonmutated Gab1 led to a strong increase of Rap1.GTP in the absence of HGF. In contrast, transfection of the GabDeltaYXXP mutant abolished the elevation of Rap1.GTP by HGF. A replating assay indicated that HGF decreases the adhesion of HEK293 cells. The results presented here delineate a novel signaling pathway from HGF to the GTPase Rap1 which depends on the interaction of the adapter protein CRKL with the exchange factor C3G and could be linked to cell migration. | 10753869
 |
Amlodipine potentiates metalloproteinase activity and accelerates elastin degradation in a model of aneurysmal disease.
J R Boyle,I M Loftus,S Goodall,M Crowther,P R Bell,M M Thompson
European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery
16
1998
显示摘要
Abdominal aortic aneurysms are characterised by changes in the extracellular matrix of the arterial media, in particular a reduction in elastin concentration. These changes are mediated by increased levels of endogenous matrix metalloproteinases (MMPs). Recently, calcium channel blockers have been shown to increase the proteolytic activity of MMP-2 secreted by vascular smooth muscle cells. It may therefore by hypothesised that calcium antagonists may potentiate the activity of MMPs in aneurysmal disease and thus accelerate AAA expansion. In this study, the ability of amlodipine--a calcium antagonist--to influence elastin degradation, was assessed in a previously described model of aneurysmal disease. | 9854552
 |
Rapid Ca2+-mediated activation of Rap1 in human platelets.
Franke, B, et al.
EMBO J., 16: 252-9 (1997)
1997
显示摘要
Rap1 is a small, Ras-like GTPase whose function and regulation are still largely unknown. We have developed a novel assay to monitor the active, GTP-bound form of Rap1 based on the differential affinity of Rap1GTP and Rap1GDP for the Rap binding domain of RalGDS (RBD). Stimulation of blood platelets with alpha-thrombin or other platelet activators caused a rapid and strong induction of Rap1 that associated with RBD in vitro. Binding to RBD increased from undetectable levels in resting platelets to >50% of total Rap1 within 30 s after stimulation. An increase in the intracellular Ca2+ concentration is both necessary and sufficient for Rap1 activation since it was induced by agents that increase intracellular Ca2+ and inhibited by a Ca2+-chelating agent. Neither inhibition of translocation of Rap1 to the cytoskeleton nor inhibition of platelet aggregation affected thrombin-induced activation of Rap1. In contrast, prostaglandin I2 (PGI2), a strong negative regulator of platelet function, inhibited agonist-induced as well as Ca2+-induced activation of Rap1. From our results, we conclude that Rap1 activation in platelets is an important common event in early agonist-induced signalling, and that this activation is mediated by an increased intracellular Ca2+ concentration. | 9029146
 |