Leptin recruits Creb-regulated transcriptional coactivator 1 to improve hyperglycemia in insulin-deficient diabetes.
Kim, GH; Szabo, A; King, EM; Ayala, J; Ayala, JE; Altarejos, JY
Molecular metabolism
4
227-36
2015
显示摘要
Leptin alleviates hyperglycemia in rodent models of Type 1 diabetes by activating leptin receptors within the central nervous system. Here we delineate whether non-canonical leptin signaling through the Creb-regulated transcriptional coactivator 1 (Crtc1) contributes to leptin-dependent improvements in diabetic glucose metabolism.We employed mice with a targeted genetic disruption of Crtc1, tracer dilution techniques and neuroanatomical studies to interrogate whether Crtc1 enables leptin to improve glucose metabolism in streptozotocin-induced (STZ) diabetes.Here we show that leptin improves diabetic glucose metabolism through Crtc1-dependent and independent mechanisms. We find that leptin reduces diabetic hyperglycemia, hepatic gluconeogenic gene expression and selectively increases glucose disposal to brown adipose tissue and heart, in STZ-diabetic Crtc1 (WT) mice but not Crtc1 (+/-) mice. By contrast, leptin decreases circulating glucagon levels in both STZ-diabetic Crtc1 (WT) and Crtc1 (+/-) mice. We also demonstrate that leptin promotes Crtc1 nuclear translocation in pro-opiomelanocortin (Pomc) and non-Pomc neurons within the hypothalamic arcuate nucleus (ARC). Accordingly, leptin's ability to induce Pomc gene expression in the ARC is blunted in STZ-diabetic Crtc1 (+/-) mice.Our study reveals that Crtc1 functions as a conduit for leptin's glucoregulatory actions in insulin-dependent diabetes. This study also highlights a new role for Crtc1 in modulating peripheral glucose metabolism. | | | 25737949
 |
Brain-derived neurotrophic factor in VMH as the causal factor for and therapeutic tool to treat visceral adiposity and hyperleptinemia in type 2 diabetic Goto-Kakizaki rats.
Maekawa, F; Fujiwara, K; Toriya, M; Maejima, Y; Nishio, T; Toyoda, Y; Nohara, K; Yashiro, T; Yada, T
Frontiers in synaptic neuroscience
5
7
2013
显示摘要
We previously reported that the type 2 diabetic Goto-Kakizaki (GK) rats at young adult ages (6-12 weeks) exhibited increased visceral fat mass and hyperleptinemia, due to hyperphagia caused primarily by neuropeptide Y (NPY) overexpression in the hypothalamic arcuate nucleus. Later, we found that GK rats continued to exhibit mesenteric fat accumulation and hyperleptinemia at least until 26 weeks of age, while hyperphagia and NPY overexpression ceased at 15 weeks of age. Therefore, we hypothesized that the long-lasting fat accumulation and hyperleptinemia are due to unidentified brain dysfunction other than NPY overexpression. In GK rats aged 26 weeks, glucose transporter-2 (GLUT2) mRNA expression in ventromedial hypothalamus (VMH) was markedly reduced in parallel with significant decreases in brain-derived neurotrophic factor (BDNF) mRNA level and BDNF-expressing cell numbers in the VMH. Pharmacologic inhibition of glucose utilization reduced BDNF mRNA expression in VMH in vivo and in vitro. The results suggested that impaired glucose utilization caused the reduction of BDNF. On the other hand, intracerebroventricular injection of BDNF for 6 days ameliorated hyperleptinemia in a long-lasting manner concurrently with feeding suppression in GK rats. Restricted feeding paired to BDNF-treated rats reduced plasma leptin level only transiently. BDNF treatment also reduced mesenteric fat mass in GK rats. These results reveal a novel action mode of BDNF to long-lastingly counteract visceral adiposity and hyperleptinemia in addition to and independently of its anorexigenic action. These results suggest that visceral fat accumulation and hyperleptinemia are at least partly due to the reduction of BDNF in VMH primarily caused by impaired glucose utilization in GK rats. The BDNF supplementation could provide an effective treatment of visceral obesity, hyperleptinemia and leptin resistance in type 2 diabetes. | | | 24106476
 |
Intracerebroventricular administration of C-type natriuretic peptide suppresses food intake via activation of the melanocortin system in mice.
Yamada-Goto, N; Katsuura, G; Ebihara, K; Inuzuka, M; Ochi, Y; Yamashita, Y; Kusakabe, T; Yasoda, A; Satoh-Asahara, N; Ariyasu, H; Hosoda, K; Nakao, K
Diabetes
62
1500-4
2013
显示摘要
C-type natriuretic peptide (CNP) and its receptor are abundantly distributed in the brain, especially in the arcuate nucleus (ARC) of the hypothalamus associated with regulating energy homeostasis. To elucidate the possible involvement of CNP in energy regulation, we examined the effects of intracerebroventricular administration of CNP on food intake in mice. The intracerebroventricular administration of CNP-22 and CNP-53 significantly suppressed food intake on 4-h refeeding after 48-h fasting. Next, intracerebroventricular administration of CNP-22 and CNP-53 significantly decreased nocturnal food intake. The increment of food intake induced by neuropeptide Y and ghrelin was markedly suppressed by intracerebroventricular administration of CNP-22 and CNP-53. When SHU9119, an antagonist for melanocortin-3 and melanocortin-4 receptors, was coadministered with CNP-53, the suppressive effect of CNP-53 on refeeding after 48-h fasting was significantly attenuated by SHU9119. Immunohistochemical analysis revealed that intracerebroventricular administration of CNP-53 markedly increased the number of c-Fos-positive cells in the ARC, paraventricular nucleus, dorsomedial hypothalamus, ventromedial hypothalamic nucleus, and lateral hypothalamus. In particular, c-Fos-positive cells in the ARC after intracerebroventricular administration of CNP-53 were coexpressed with α-melanocyte-stimulating hormone immunoreactivity. These results indicated that intracerebroventricular administration of CNP induces an anorexigenic action, in part, via activation of the melanocortin system. | | | 23274904
 |
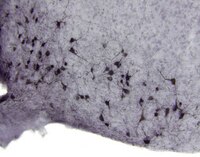
IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes.
Li, J; Tang, Y; Cai, D
Nature cell biology
14
999-1012
2012
显示摘要
Adult neural stem cells (NSCs) are known to exist in a few regions of the brain; however, the entity and physiological/disease relevance of adult hypothalamic NSCs (htNSCs) remain unclear. This work shows that adult htNSCs are multipotent and predominantly present in the mediobasal hypothalamus of adult mice. Chronic high-fat-diet feeding led to not only depletion but also neurogenic impairment of htNSCs associated with IKKβ/NF-κB activation. In vitro htNSC models demonstrated that their survival and neurogenesis markedly decreased on IKKβ/NF-κB activation but increased on IKKβ/NF-κB inhibition, mechanistically mediated by IKKβ/NF-κB-controlled apoptosis and Notch signalling. Mouse studies revealed that htNSC-specific IKKβ/NF-κB activation led to depletion and impaired neuronal differentiation of htNSCs, and ultimately the development of obesity and pre-diabetes. In conclusion, adult htNSCs are important for the central regulation of metabolic physiology, and IKKβ/NF-κB-mediated impairment of adult htNSCs is a critical neurodegenerative mechanism for obesity and related diabetes. | Immunofluorescence | | 22940906
 |
DLK1 is a somato-dendritic protein expressed in hypothalamic arginine-vasopressin and oxytocin neurons.
Villanueva, C; Jacquier, S; de Roux, N
PloS one
7
e36134
2012
显示摘要
Delta-Like 1 Homolog, Dlk1, is a paternally imprinted gene encoding a transmembrane protein involved in the differentiation of several cell types. After birth, Dlk1 expression decreases substantially in all tissues except endocrine glands. Dlk1 deletion in mice results in pre-natal and post-natal growth deficiency, mild obesity, facial abnormalities, and abnormal skeletal development, suggesting involvement of Dlk1 in perinatal survival, normal growth and homeostasis of fat deposition. A neuroendocrine function has also been suggested for DLK1 but never characterised. To evaluate the neuroendocrine function of DLK1, we first characterised Dlk1 expression in mouse hypothalamus and then studied post-natal variations of the hypothalamic expression. Western Blot analysis of adult mouse hypothalamus protein extracts showed that Dlk1 was expressed almost exclusively as a soluble protein produced by cleavage of the extracellular domain. Immunohistochemistry showed neuronal DLK1 expression in the suprachiasmatic (SCN), supraoptic (SON), paraventricular (PVN), arcuate (ARC), dorsomedial (DMN) and lateral hypothalamic (LH) nuclei. DLK1 was expressed in the dendrites and perikarya of arginine-vasopressin neurons in PVN, SCN and SON and in oxytocin neurons in PVN and SON. These findings suggest a role for DLK1 in the post-natal development of hypothalamic functions, most notably those regulated by the arginine-vasopressin and oxytocin systems. | | | 22563444
 |
Continuous stress-induced dopamine dysregulation augments PAP-I and PAP-II expression in melanotrophs of the pituitary gland.
Konishi H, Ogawa T, Kawahara S, Matsumoto S, Kiyama H
Biochem Biophys Res Commun
2011
显示摘要
Under continuous stress (CS) in rats, melanotrophs, the predominant cell-type in the intermediate lobe (IL) of the pituitary, are hyperactivated to secrete α-melanocyte-stimulating hormone and thereafter degenerate. Although these phenomena are drastic, the molecular mechanisms underlying the cellular changes are mostly unknown. In this study, we focused on the pancreatitis-associated protein (PAP) family members of the secretory lectins and characterized their expression in the IL of CS model rats because we had identified two members of this family as up-regulated genes in our previous microarray analysis. RT-PCR and histological studies demonstrated that prominent PAP-I and PAP-II expression was induced in melanotrophs in the early stages of CS, while another family member, PAP-III, was not expressed. We further examined the regulatory mechanisms of PAP-I and PAP-II expression and revealed that both were induced by the decreased dopamine levels in the IL under CS. Because the PAP family members are implicated in cell survival and proliferation, PAP-I and PAP-II secreted from melanotrophs may function to sustain homeostasis of the IL under CS conditions in an autocrine or a paracrine manner.Copyright © 2011. Published by Elsevier Inc. | | | 21329657
 |
Id1, Id2 and Id3 are induced in rat melanotrophs of the pituitary gland by dopamine suppression under continuous stress.
Konishi H, Ogawa T, Nakagomi S, Inoue K, Tohyama M, Kiyama H
Neuroscience
169
1527-34. Epub 2010 Jun 20.
2010
显示摘要
In rats under continuous stress (CS) there is decreased hypothalamic dopaminergic innervation to the intermediate lobe (IL) of the pituitary gland, which causes hyperactivation and subsequent degeneration of melanotrophs in the IL. In this study, we investigated the molecular basis for the changes that occur in melanotrophs during CS. Using microarray analysis, we identified several genes differentially expressed in the IL under CS conditions. Among the genes up-regulated under CS conditions, we focused on the inhibitor of DNA binding/differentiation (Id) family of dominant negative basic helix-loop-helix (bHLH) transcription factors. RT-PCR, Western blotting and in situ hybridization confirmed the significant inductions of Id1, Id2 and Id3 in the IL of CS rats. Administration of the dopamine D2 receptor agonist bromocriptine prevented the inductions of Id1-3 in the IL of CS rats, whereas application of the dopamine D2 antagonist sulpiride induced significant expressions of Id1-3 in the IL of normal rats. Moreover, an in vitro study using primary cultured melanotrophs demonstrated a direct effect on Id1-3 inductions by dopamine suppression. These results suggest that the decreased dopamine levels in the IL during CS induce Id1-3 expressions in melanotrophs. Because Id family members inhibit various bHLH transcription factors, it is conceivable that the induced Id1-3 would cooperatively modulate gene expressions in melanotrophs under CS conditions to induce hormone secretion. | | | 20600660
 |
P2X2 purinoreceptor protein in hypothalamic neurons associated with the regulation of food intake.
Colldén G, Mangano C, Meister B
Neuroscience
171
62-78. Epub 2010 Aug 22.
2010
显示摘要
Purines such as ATP act as extracellular messengers through specific purinergic receptors. Three different classes of purinergic receptors have been identified and termed P1, P2X and P2Y. The purinergic receptor subunit P2X2 is a ligand-gated ion channel that is widely expressed by neurons in the central nervous system. The aim of this study was to study the cellular localization and to identify the chemical phenotypes of ionotropic P2X2 receptor (P2X2R)-containing neurons in the rat mediobasal hypothalamus by immunohistochemistry using three different P2X2R antisera, with special reference to neurons that influence food intake and body weight. P2X2R immunoreactivity was mainly observed in cell bodies and neural extensions located in the ventromedial part of the hypothalamic arcuate nucleus, a subregion of the nucleus with a weak blood-brain barrier (BBB). At the subcellular level, P2X2R immunoreactivity was located to the periphery of individual cells, likely representing the plasma membrane. Many P2X2R-immunoreactive cell bodies in the arcuate nucleus contained the orexigenic peptides neuropeptide Y (NPY) and agouti-related protein (AgRP), and the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD). In contrast, P2X2R immunoreactive cell bodies of the arcuate nucles only occasionally contained the anorexigenic peptides α-melanocyte-stimulating hormone (α-MSH) or cocaine- and amphetamine-regulated transcript (CART), or the opioid peptide dynorphin (DYN). There was no evidence for colocalization of P2X2R with somatostatin or neuronal nitric oxide synthase (nNOS) in neurons of the arcuate nucleus. In the parvocellular part of the paraventricular nucleus, P2X2R was demonstrated in some corticotropin-releasing hormone (CRH), thyrotropin-releasing hormone (TRH) and CART-containing neurons. In some cell bodies of the lateral hypothalamic area P2X2R was colocalized with DYN. The presence of P2X2R immunoreactivity in primarily orexigenic NPY/AgRP/GABA-containing neurons of the arcuate nucleus suggests that extracellular ATP has a regulatory action on this neuronal population located in a strategic position of the brain. | | | 20736052
 |
Nociceptin/orphanin FQ peptide in hypothalamic neurones associated with the control of feeding behaviour.
Maolood N, Meister B
J Neuroendocrinol
22
75-82. Epub 2009 Dec 15.
2010
显示摘要
Nociceptin/orphanin FQ (N/OFQ), an endogenous peptide agonist of the opioid N/OFQ receptor, has been implicated in the regulation of energy balance. In the present study, we have used immunohistochemistry to investigate the cellular localisation and colocalisation of N/OFQ-immunoreactive cell bodies in hypothalamic regions containing neurones producing orexigenic or anorexigenic transmitters. In colchicine-treated rats, N/OFQ immunoreactivity was demonstrated in many cell bodies of the arcuate nucleus (Arc), paraventricular nucleus (PVN) and lateral hypothalamic area (LHA). Double-labelling revealed that N/OFQ was present in some neurones located in the ventrolateral part of the Arc producing pro-opiomelanocortin, as shown by the presence of the anorexigenic peptides alpha-melanocyte-stimulating hormone (alpha-MSH) and cocaine- and amphetamine-regulated transcript and, occasionally, in single neurones of the ventrolateral Arc producing orexigenic agouti-related peptide, but not neuropeptide Y. N/OFQ immunoreactivity was also demonstrated in a few tyrosine hydroxylase- or dynorphin (DYN)-containing neurones in the dorsomedial part of the Arc. In the parvocellular PVN, N/OFQ was demonstrated in some thyrotrophin-releasing hormone- or DYN-, but not corticotrophin-releasing hormone-containing neurones. Most N/OFQ-immunoreactive neurones in the LHA contained orexin- and DYN, but not melanin-concentrating hormone. The results obtained, demonstrating the presence of N/OFQ in some alpha-MSH- and in many orexin-containing neurones, suggest a functional relationship between these neuropeptides and N/OFQ in the control of feeding behaviour and body weight. | | | 20025627
 |
Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus.
P Ciofi, M Garret, O Lapirot, P Lafon, A Loyens, V Prevot, JE Levine
Endocrinology
150
5509-19
2009
显示摘要
Blood-borne hormones acting in the mediobasal hypothalamus, like those controlling food intake, require relatively direct access to target chemosensory neurons of the arcuate nucleus (ARC). An anatomical substrate for this is a permeable microvasculature with fenestrated endothelial cells in the ARC, a system that has awaited comprehensive documentation. Here, the immunofluorescent detection of endothelial fenestral diaphragms in the rat ARC allowed us to quantitate permeable microvessels throughout its rostrocaudal extent. We have determined that permeable microvessels are part of the subependymal plexus irrigating exclusively the ventromedial (vm) ARC from the subadjacent neuroendocrine median eminence. Unexpectedly, permeable microvessels were concentrated proximal to the pituitary stalk. This marked topography strongly supports the functional importance of retrograde blood flow from the pituitary to the vmARC, therefore making a functional relationship between peripheral long-loop, pituitary short-loop, and neuroendocrine ultra-short loop feedback, altogether converging for integration in the vmARC (formerly known as the hypophysiotrophic area), thereby so pivotal as a multicompetent brain endocrinostat. 全文本文章 | | | 19837874
 |