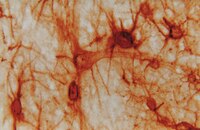
The identification of proteoglycans and glycosaminoglycans in archaeological human bones and teeth.
Coulson-Thomas, YM; Coulson-Thomas, VJ; Norton, AL; Gesteira, TF; Cavalheiro, RP; Meneghetti, MC; Martins, JR; Dixon, RA; Nader, HB
PloS one
10
e0131105
2015
显示摘要
Bone tissue is mineralized dense connective tissue consisting mainly of a mineral component (hydroxyapatite) and an organic matrix comprised of collagens, non-collagenous proteins and proteoglycans (PGs). Extracellular matrix proteins and PGs bind tightly to hydroxyapatite which would protect these molecules from the destructive effects of temperature and chemical agents after death. DNA and proteins have been successfully extracted from archaeological skeletons from which valuable information has been obtained; however, to date neither PGs nor glycosaminoglycan (GAG) chains have been studied in archaeological skeletons. PGs and GAGs play a major role in bone morphogenesis, homeostasis and degenerative bone disease. The ability to isolate and characterize PG and GAG content from archaeological skeletons would unveil valuable paleontological information. We therefore optimized methods for the extraction of both PGs and GAGs from archaeological human skeletons. PGs and GAGs were successfully extracted from both archaeological human bones and teeth, and characterized by their electrophoretic mobility in agarose gel, degradation by specific enzymes and HPLC. The GAG populations isolated were chondroitin sulfate (CS) and hyaluronic acid (HA). In addition, a CSPG was detected. The localization of CS, HA, three small leucine rich PGs (biglycan, decorin and fibromodulin) and glypican was analyzed in archaeological human bone slices. Staining patterns were different for juvenile and adult bones, whilst adolescent bones had a similar staining pattern to adult bones. The finding that significant quantities of PGs and GAGs persist in archaeological bones and teeth opens novel venues for the field of Paleontology. | | | 26107959
 |
Local Delivery of High-Dose Chondroitinase ABC in the Sub-Acute Stage Promotes Axonal Outgrowth and Functional Recovery after Complete Spinal Cord Transection.
Cheng, CH; Lin, CT; Lee, MJ; Tsai, MJ; Huang, WH; Huang, MC; Lin, YL; Chen, CJ; Huang, WC; Cheng, H
PloS one
10
e0138705
2015
显示摘要
Chondroitin sulfate proteoglycans (CSPGs) are glial scar-associated molecules considered axonal regeneration inhibitors and can be digested by chondroitinase ABC (ChABC) to promote axonal regeneration after spinal cord injury (SCI). We previously demonstrated that intrathecal delivery of low-dose ChABC (1 U) in the acute stage of SCI promoted axonal regrowth and functional recovery. In this study, high-dose ChABC (50 U) introduced via intrathecal delivery induced subarachnoid hemorrhage and death within 48 h. However, most SCI patients are treated in the sub-acute or chronic stages, when the dense glial scar has formed and is minimally digested by intrathecal delivery of ChABC at the injury site. The present study investigated whether intraparenchymal delivery of ChABC in the sub-acute stage of complete spinal cord transection would promote axonal outgrowth and improve functional recovery. We observed no functional recovery following the low-dose ChABC (1 U or 5 U) treatments. Furthermore, animals treated with high-dose ChABC (50 U or 100 U) showed decreased CSPGs levels. The extent and area of the lesion were also dramatically decreased after ChABC treatment. The outgrowth of the regenerating axons was significantly increased, and some partially crossed the lesion site in the ChABC-treated groups. In addition, retrograde Fluoro-Gold (FG) labeling showed that the outgrowing axons could cross the lesion site and reach several brain stem nuclei involved in sensory and motor functions. The Basso, Beattie and Bresnahan (BBB) open field locomotor scores revealed that the ChABC treatment significantly improved functional recovery compared to the control group at eight weeks after treatment. Our study demonstrates that high-dose ChABC treatment in the sub-acute stage of SCI effectively improves glial scar digestion by reducing the lesion size and increasing axonal regrowth to the related functional nuclei, which promotes locomotor recovery. Thus, our results will aid in the treatment of spinal cord injury. | | | 26393921
 |
Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment.
Silver, DJ; Siebzehnrubl, FA; Schildts, MJ; Yachnis, AT; Smith, GM; Smith, AA; Scheffler, B; Reynolds, BA; Silver, J; Steindler, DA
The Journal of neuroscience : the official journal of the Society for Neuroscience
33
15603-17
2013
显示摘要
Glioblastoma (GBM) remains the most pervasive and lethal of all brain malignancies. One factor that contributes to this poor prognosis is the highly invasive character of the tumor. GBM is characterized by microscopic infiltration of tumor cells throughout the brain, whereas non-neural metastases, as well as select lower grade gliomas, develop as self-contained and clearly delineated lesions. Illustrated by rodent xenograft tumor models as well as pathological human patient specimens, we present evidence that one fundamental switch between these two distinct pathologies--invasion and noninvasion--is mediated through the tumor extracellular matrix. Specifically, noninvasive lesions are associated with a rich matrix containing substantial amounts of glycosylated chondroitin sulfate proteoglycans (CSPGs), whereas glycosylated CSPGs are essentially absent from diffusely infiltrating tumors. CSPGs, acting as central organizers of the tumor microenvironment, dramatically influence resident reactive astrocytes, inducing their exodus from the tumor mass and the resultant encapsulation of noninvasive lesions. Additionally, CSPGs induce activation of tumor-associated microglia. We demonstrate that the astrogliotic capsule can directly inhibit tumor invasion, and its absence from GBM presents an environment favorable to diffuse infiltration. We also identify the leukocyte common antigen-related phosphatase receptor (PTPRF) as a putative intermediary between extracellular glycosylated CSPGs and noninvasive tumor cells. In all, we present CSPGs as critical regulators of brain tumor histopathology and help to clarify the role of the tumor microenvironment in brain tumor invasion. | Immunohistochemistry | | 24068827
 |
PDGF suppresses the sulfation of CD44v and potentiates CD44v-mediated binding of colon carcinoma cells to fibrin under flow.
Alves, CS; Konstantopoulos, K
PloS one
7
e41472
2012
显示摘要
Fibrin(ogen) mediates sustained tumor cell adhesion and survival in the pulmonary vasculature, thereby facilitating the metastatic dissemination of tumor cells. CD44 is the major functional fibrin receptor on colon carcinoma cells. Growth factors, such as platelet-derived growth factor (PDGF), induce post-translational protein modifications, which modulate ligand binding activity. In view of the roles of PDGF, fibrin(ogen) and CD44 in cancer metastasis, we aimed to delineate the effect of PDGF on CD44-fibrin recognition. By immunoprecipitating CD44 from PDGF-treated and untreated LS174T colon carcinoma cells, which express primarily CD44v, we demonstrate that PDGF enhances the adhesion of CD44v-coated beads to immobilized fibrin. Enzymatic inhibition studies coupled with flow-based adhesion assays and autoradiography reveal that PDGF augments the binding of CD44v to fibrin by significantly attenuating the extent of CD44 sulfation primarily on chondroitin and dermatan sulfate chains. Surface plasmon resonance assays confirm that PDGF enhances the affinity of CD44v-fibrin binding by markedly reducing its dissociation rate while modestly increasing the association rate. PDGF mildly reduces the affinity of CD44v-hyaluronan binding without affecting selectin-CD44v recognition. The latter is attributed to the fact that CD44v binds to selectins via sialofucosylated O-linked residues independent of heparan, dermatan and chondroitin sulfates. Interestingly, PDGF moderately reduces the sulfation of CD44s and CD44s-fibrin recognition. Collectively, these data offer a novel perspective into the mechanism by which PGDF regulates CD44-dependent binding of metastatic colon carcinoma cells to fibrin(ogen). | | | 23056168
 |
Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice.
Yi, JH; Katagiri, Y; Susarla, B; Figge, D; Symes, AJ; Geller, HM
The Journal of comparative neurology
520
3295-313
2012
显示摘要
Chondroitin sulfate proteoglycans (CSPGs) play a pivotal role in many neuronal growth mechanisms including axon guidance and the modulation of repair processes following injury to the spinal cord or brain. Many actions of CSPGs in the central nervous system (CNS) are governed by the specific sulfation pattern on the glycosaminoglycan (GAG) chains attached to CSPG core proteins. To elucidate the role of CSPGs and sulfated GAG chains following traumatic brain injury (TBI), controlled cortical impact injury of mild to moderate severity was performed over the left sensory motor cortex in mice. Using immunoblotting and immunostaining, we found that TBI resulted in an increase in the CSPGs neurocan and NG2 expression in a tight band surrounding the injury core, which overlapped with the presence of 4-sulfated CS GAGs but not with 6-sulfated GAGs. This increase was observed as early as 7 days post injury (dpi), and persisted for up to 28 dpi. Labeling with markers against microglia/macrophages, NG2+ cells, fibroblasts, and astrocytes showed that these cells were all localized in the area, suggesting multiple origins of chondroitin-4-sulfate increase. TBI also caused a decrease in the expression of aggrecan and phosphacan in the pericontusional cortex with a concomitant reduction in the number of perineuronal nets. In summary, we describe a dual response in CSPGs whereby they may be actively involved in complex repair processes following TBI. | Immunofluorescence | Mouse | 22628090
 |
Gel structure has an impact on pericellular and extracellular matrix deposition, which subsequently alters metabolic activities in chondrocyte-laden PEG hydrogels.
Nicodemus, GD; Skaalure, SC; Bryant, SJ
Acta biomaterialia
7
492-504
2011
显示摘要
While designing poly(ethylene glycol) hydrogels with high moduli suitable for in situ placement is attractive for cartilage regeneration, the impact of a tighter crosslinked structure on the organization and deposition of the matrix is not fully understood. The objectives of this study were to characterize the composition and spatial organization of new matrix as a function of gel crosslinking and study its impact on chondrocytes in terms of anabolic and catabolic gene expression and catabolic activity. Bovine articular chondrocytes were encapsulated in hydrogels with three crosslinking densities (compressive moduli 60, 320 and 590 kPa) and cultured for 25 days. Glycosaminoglycan production increased with culture time and was greatest in the gels with lowest crosslinking. Collagens II and VI, aggrecan, link protein and decorin were localized to pericellular regions in all gels, but their presence decreased with increasing gel crosslinking. Collagen II and aggrecan expression were initially up-regulated in gels with higher crosslinking, but increased similarly up to day 15. Matrix metalloproteinase (MMP)-1 and MMP-13 expression were elevated (∼25-fold) in gels with higher crosslinking throughout the study, while MMP-3 was unaffected by gel crosslinking. The presence of aggrecan and collagen degradation products confirmed MMP activity. These findings indicate that chondrocytes synthesized the major cartilage components within PEG hydrogels, however, gel structure had a significant impact on the composition and spatial organization of the new tissue and on how chondrocytes responded to their environment, particularly with respect to their catabolic expression. | | | 20804868
 |
Chondroitin-4-sulfation negatively regulates axonal guidance and growth.
Wang, H; Katagiri, Y; McCann, TE; Unsworth, E; Goldsmith, P; Yu, ZX; Tan, F; Santiago, L; Mills, EM; Wang, Y; Symes, AJ; Geller, HM
Journal of cell science
121
3083-91
2008
显示摘要
Glycosaminoglycan (GAG) side chains endow extracellular matrix proteoglycans with diversity and complexity based upon the length, composition and charge distribution of the polysaccharide chain. Using cultured primary neurons, we show that specific sulfation in the GAG chains of chondroitin sulfate mediates neuronal guidance cues and axonal growth inhibition. Chondroitin-4-sulfate (CS-A), but not chondroitin-6-sulfate (CS-C), exhibits a strong negative guidance cue to mouse cerebellar granule neurons. Enzymatic and gene-based manipulations of 4-sulfation in the GAG side chains alter their ability to direct growing axons. Furthermore, 4-sulfated chondroitin sulfate GAG chains are rapidly and significantly increased in regions that do not support axonal regeneration proximal to spinal cord lesions in mice. Thus, our findings show that specific sulfation along the carbohydrate backbone carries instructions to regulate neuronal function. 全文本文章 | | | 18768934
 |
Adult bone marrow-derived mononuclear cells expressing chondroitinase AC transplanted into CNS injury sites promote local brain chondroitin sulphate degradation.
Yvette M Coulson-Thomas,Vivien J Coulson-Thomas,Thais R Filippo,Renato A Mortara,Rafael B da Silveira,Helena B Nader,Marimélia A Porcionatto
Journal of neuroscience methods
171
2008
显示摘要
Injury to the CNS of vertebrates leads to the formation of a glial scar and production of inhibitory molecules, including chondroitin sulphate proteoglycans. Various studies suggest that the sugar component of the proteoglycan is responsible for the inhibitory role of these compounds in axonal regeneration. By degrading chondroitin sulphate chains with specific enzymes, denominated chondroitinases, the inhibitory capacity of these proteoglycans is decreased. Chondroitinase administration involves frequent injections of the enzyme at the lesion site which constitutes a rather invasive method. We have produced a vector containing the gene for Flavobacterium heparinum chondroitinase AC for expression in adult bone marrow-derived cells which were then transplanted into an injury site in the CNS. The expression and secretion of active chondroitinase AC was observed in vitro using transfected Chinese hamster ovarian and gliosarcoma cells and in vivo by immunohistochemistry analysis which showed degraded chondroitin sulphate coinciding with the location of transfected bone marrow-derived cells. Immunolabelling of the axonal growth-associated protein GAP-43 was observed in vivo and coincided with the location of degraded chondroitin sulphate. We propose that bone marrow-derived mononuclear cells, transfected with our construct and transplanted into CNS, could be a potential tool for studying an alternative chondroitinase AC delivery method. | | | 18417222
 |
Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans.
Laabs, TL; Wang, H; Katagiri, Y; McCann, T; Fawcett, JW; Geller, HM
The Journal of neuroscience : the official journal of the Society for Neuroscience
27
14494-501
2007
显示摘要
Chondroitin sulfate proteoglycans (CSPGs) are upregulated in the CNS after injury and participate in the inhibition of axon regeneration mainly through their glycosaminoglycan (GAG) side chains. In the present study, we have identified a new way to alleviate the inhibition of axonal regeneration by CSPG GAGs. We have successfully decreased the amount of CSPG GAG produced by astrocytes by targeting chondroitin polymerizing factor (ChPF), a key enzyme in the CSPG biosynthetic pathway. Using short interfering RNA (siRNA), we reduced ChPF mRNA levels by 70% in both the Neu7 astrocyte cell line and primary rat astrocytes. This reduction leads to a decrease in ChPF protein levels and a reduced amount of CSPG GAG chains in the conditioned media (CM) of these cells. Secretion of neurocan by primary astrocytes and NG2 core protein by Neu7 cells transfected with ChPF siRNA is not decreased, suggesting that inhibiting GAG chain synthesis does not affect core protein trafficking from these cells. CM from siRNA-treated Neu7 cells is a less repulsive substrate for axons than CM from control cells. In addition, axonal outgrowth from cerebellar granule neurons is increased on or in CM from ChPF siRNA-treated Neu7 cells. These data indicate that targeting the biosynthesis of CSPG GAG is a potentially new therapeutic avenue for decreasing CSPG GAG produced by astrocytes after CNS injury. | | | 18160657
 |
Chondroitinase applied to peripheral nerve repair averts retrograde axonal regeneration.
Graham, JB; Neubauer, D; Xue, QS; Muir, D
Experimental neurology
203
185-95
2007
显示摘要
Antegrade, target-directed axonal regeneration is the explicit goal of nerve repair. However, aberrant and dysfunctional regrowth is commonly observed as well. At the site of surgical nerve coaptation, axonal sprouts encounter fibrotic connective tissue rich in growth-inhibiting chondroitin sulfate proteoglycan that may contribute to misdirection of axonal regrowth. In the present study, we tested the hypothesis that degradation of chondroitin sulfate proteoglycan by application of chondroitinase at the site of nerve repair can decrease aberrant axonal growth. Adult rats received bilateral sciatic nerve transection and end-to-end repair. One nerve was injected with chondroitinase ABC and the contralateral nerve treated with vehicle alone. After 28 weeks, retrograde axonal regeneration was assessed proximal to the repair by scoring neurofilament-immunopositive axons within the nerve (intrafascicular) and outside the nerve proper (extrafascicular). Intrafascicular retrograde axonal growth was equivalent in both control and chondroitinase treatment conditions. In contrast, chondroitinase treatment caused a pronounced (93%) reduction in extrafascicular retrograde axonal growth. The decrease in axon egress from the nerve was coincident with an increase in antegrade regeneration and improved recovery of motor function. Based on these findings, we conclude that chondroitinase applied at the site of nerve transection repair averts dysfunctional extrafascicular retrograde axonal growth. 全文本文章 | | | 16970940
 |