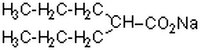676380 Sigma-AldrichValproic Acid, Sodium Salt - CAS 1069-66-5 - Calbiochem
Valproic Acid, Sodium Salt, CAS 1069-66-5, is a cell-permeable inhibitor of histone deacetylase (IC50 = 400 µM for HDAC1). Inhibits proliferation of human malignant glioma cell lines.
More>> Valproic Acid, Sodium Salt, CAS 1069-66-5, is a cell-permeable inhibitor of histone deacetylase (IC50 = 400 µM for HDAC1). Inhibits proliferation of human malignant glioma cell lines. Less<<Synonyms: 2-Propylpentanoic Acid, Na
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 1069-66-5 | C₈H₁₅O₂Na |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 676380-5GMCN | Plastic ampoule | 5 gm |
| Product Information | |
|---|---|
| CAS number | 1069-66-5 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₈H₁₅O₂Na |
| Chemical formula | C₈H₁₅O₂Na |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Primary Target | HDAC1 |
| Primary Target IC<sub>50</sub> | 400 µM |
| Purity | ≥98% by GC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | YV7876000 |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 676380-5GMCN | 04055977183566 |
Documentation
Valproic Acid, Sodium Salt - CAS 1069-66-5 - Calbiochem SDS
| Title |
|---|
Valproic Acid, Sodium Salt - CAS 1069-66-5 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 676380 |
References
| Reference overview |
|---|
| Gottlicher, M., et al. 2001. EMBO J. 20, 6969. Knupfer, M.M., et al. 2001. Anticancer Res. 21, 347. Phiel, C.J., et al. 2001. J. Biol. Chem. 276, 36734. Vaden, D.L., et al. 2001. J. Biol. Chem. 276, 15466. Yuan, P.X., et al. 2001. J. Biol. Chem. 276, 31674. Chen, G., et al. 1999. J. Neurochem. 72, 1327. |
Technical Info
| Title |
|---|
| White Paper - The Message in the Marks: Deciphering Cancer Epigenetics |
Data Sheet
| Title |
|---|
| Reprogramming Cell Fate and Function Novel Strategies for iPSC Generation, Characterization, and Differentiation |