496915 Sigma-AldrichOridonin, R. rubescens - CAS 28957-04-2 - Calbiochem
A cell-permeable diterpenoid compound that possesses anti NF-κB activity and displays antiproliferative and antiangiogenic properties.
More>> A cell-permeable diterpenoid compound that possesses anti NF-κB activity and displays antiproliferative and antiangiogenic properties. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
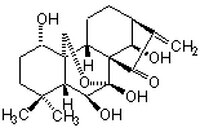
| 28957-04-2 | C₂₀H₂₈O₆ |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 496915-5MGCN | Plastic ampoule | 5 mg |
| References | |
|---|---|
| References | Ikezoe, T., et al. 2005. Mol. Cancer Ther. 4, 578. Meade-Tollin, L.C., et al. 2004. J. Nat. Prod. 67, 2. |
| Product Information | |
|---|---|
| CAS number | 28957-04-2 |
| ATP Competitive | N |
| Form | Yellow solid |
| Hill Formula | C₂₀H₂₈O₆ |
| Chemical formula | C₂₀H₂₈O₆ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | NF-κB transcriptional activity |
| Primary Target IC<sub>50</sub> | 5 µg/ml inhibiting p65 NF-κB transcriptional activity in MT-1 cells |
| Purity | ≥93% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | NZ8177000 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 496915-5MGCN | 04055977199871 |
Documentation
Oridonin, R. rubescens - CAS 28957-04-2 - Calbiochem SDS
| Title |
|---|
Oridonin, R. rubescens - CAS 28957-04-2 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 496915 |
References
| Reference overview |
|---|
| Ikezoe, T., et al. 2005. Mol. Cancer Ther. 4, 578. Meade-Tollin, L.C., et al. 2004. J. Nat. Prod. 67, 2. |