422175 Sigma-AldrichL-165,041 - CAS 79558-09-1 - Calbiochem
A cell-permeable phenoxyacetic acid derivative that acts as a potent and selective peroxisome proliferator activator receptor δ (PPARδ) agonist (Ki = 6 nM for hPPARδ and 730 nM for hPPARγ).
More>> A cell-permeable phenoxyacetic acid derivative that acts as a potent and selective peroxisome proliferator activator receptor δ (PPARδ) agonist (Ki = 6 nM for hPPARδ and 730 nM for hPPARγ). Less<<Synonyms: Compound P, L165041, 4-[3-(2-Propyl-3-hydroxy-4-acetyl)phenoxy]propyloxyphenoxy-acetic acid, PPAR Agonist VIII, PPARα Agonist III, PPARγ Agonist VII
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 79558-09-1 | C₂₂H₂₆O₇ |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 422175-5MGCN | Plastic ampoule | 5 mg |
| Product Information | |
|---|---|
| CAS number | 79558-09-1 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₂₂H₂₆O₇ |
| Chemical formula | C₂₂H₂₆O₇ |
| Reversible | N |
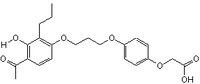
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Peroxisome proliferator activator receptor δ (PPAR@delta;) |
| Primary Target K<sub>i</sub> | 6 nM for hPPARδ and 730 nM for hPPARγ |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 422175-5MGCN | 04055977187144 |
Documentation
L-165,041 - CAS 79558-09-1 - Calbiochem SDS
| Title |
|---|
L-165,041 - CAS 79558-09-1 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 422175 |
References
| Reference overview |
|---|
| Rival, Y., et al. 2002. Eur. J. Pharmacol. 435, 143. Castrillo, A., et al. 2001. J. Biol. Chem. 276, 34082. Hansen, J.B., et al. 2001. J. Biol. Chem. 276, 3175. Son, C., et al. 2001. Endocrinology 142, 4189. Wilkie, N., et al. 2001. J. Neurochem. 78, 1135. Leibowitz, M.D., et al. 2000. FEBS Lett. 473, 333. Berger, J., et al. 1999. J. Biol. Chem. 274, 6718. Lim, H., et al. 1999. Genes Dev. 13, 1561. |