438187 Sigma-AldrichInSolution™ Lovastatin, Sodium Salt - Calbiochem
Carboxylate form of Lovastatin that is active in whole cells and cell-free assays.
More>> Carboxylate form of Lovastatin that is active in whole cells and cell-free assays. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₂₄H₃₇O₆ • Na |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 438187-5MGCN | Glass bottle | 5 mg |
| Description | |
|---|---|
| Overview | Carboxylate form of Lovastatin (Cat. No. 438185) that is active in whole cells and cell-free assays. Lovastatin is reported to be an anti-hypercholesterolemic agent that acts as a reversible competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and blocks a series of biological events including activation of p21ras by insulin in 3T3-L1 fibroblasts and 3T3-L1 adipocytes, farnesylation of p21ras, which decreases the pool of intracellular Ras available for subsequent activation by growth factors (including insulin), and N-ras-induced neuronal differentiation. Causes cell cycle arrest in the late G1 phase. Shown to inhibit the stimulation of MAP kinase by insulin in HIRcB cells and block the transcription of the type-I SCR gene in THP-1-derived macrophages. Also blocks PDGF receptor tyrosine phosphorylation and MAP kinase activation by PDGF. |
| Catalogue Number | 438187 |
| Brand Family | Calbiochem® |
| Product Information | |
|---|---|
| Form | Liquid |
| Formulation | A 10 mM (5 mg/1.13 ml) solution of Lovastatin, Sodium Salt (Cat. No. 438186) in H₂O. |
| Hill Formula | C₂₄H₃₇O₆ • Na |
| Chemical formula | C₂₄H₃₇O₆ • Na |
| Reversible | Y |
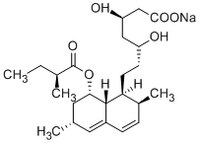
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | HMG-CoA reductase |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information | |
|---|---|
| S Phrase | S: 24/25 Avoid contact with skin and eyes. |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 438187-5MGCN | 04055977186994 |
Documentation
InSolution™ Lovastatin, Sodium Salt - Calbiochem SDS
| Title |
|---|
InSolution™ Lovastatin, Sodium Salt - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 438187 |
References
| Reference overview |
|---|
| Rao, S., et al. 1999. Proc. Natl. Acad. Sci. USA 96, 7197. Carel, K., et al. 1996. J. Biol. Chem. 271, 30625. McGuire, T.F., et al. 1996. J. Biol. Chem. 271, 27402. Umetani, N., et al. 1996. Biochim. Biophys. Acta 1303, 199. Xu, X.Q., et al. 1996. Arch. Biochem. Biophys. 326, 233. Reusch, J.E.-B., et al. 1995. J. Biol. Chem. 270, 2036. |
| Data Sheet | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|